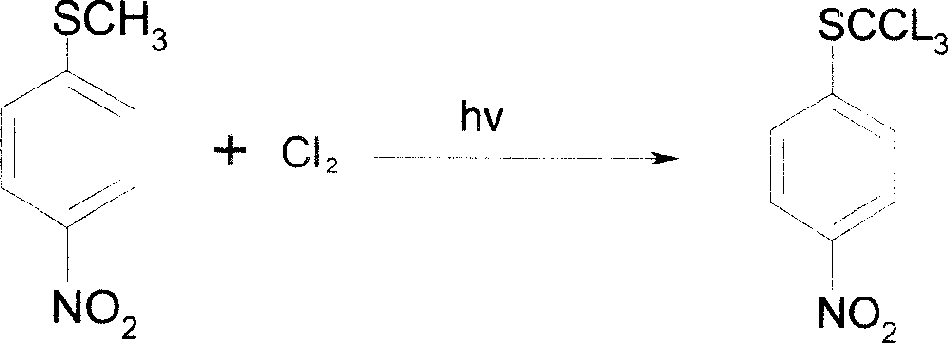Method of producing toltrazuril
A technology of toltrazuril and reaction, applied in the direction of organic chemistry, etc., can solve problems such as increased drug resistance of coccidia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
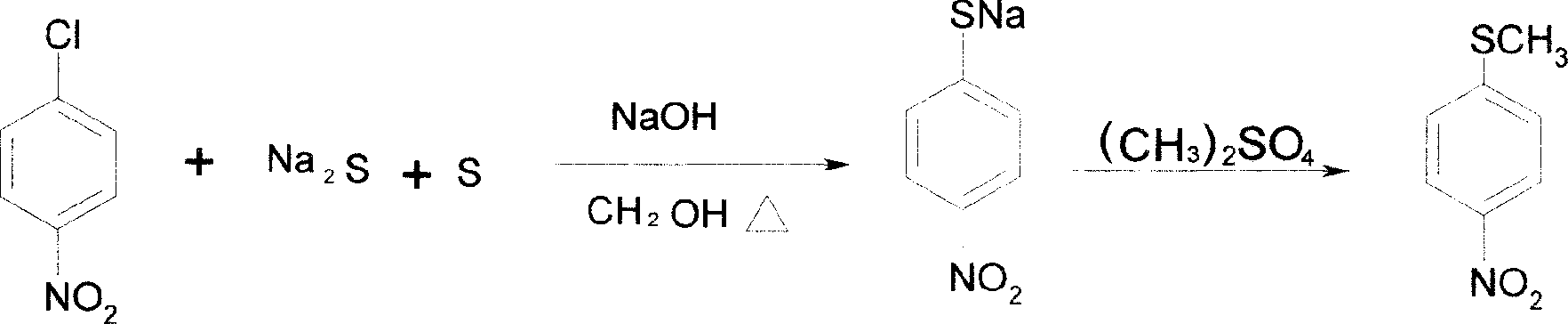
[0040] The preparation process of toltrazuril:
[0041] Reaction 1: Mix 410g of methanol and 158g of p-nitrochlorobenzene to fully melt, and add dropwise a mixture of sulfur, sodium sulfide and methanol. Keep at 60-65°C for 2 hours, cool down, add 880g of water, let cool, add 192g of dimethyl sulfate dropwise, and adjust the pH to greater than 9 with sodium hydroxide solution. After the reaction, 153.6 g of methyl sulfide was obtained by spin filtration. The yield was 91.1%.
[0042] Reaction 2: 1800g of chloroform and 400g of methyl sulfide, stir evenly, control the temperature at 75-80°C, let the chlorine gas react for about 3 hours, and filter to obtain 600.9g of chlorinated compounds. The yield was 92.7%.
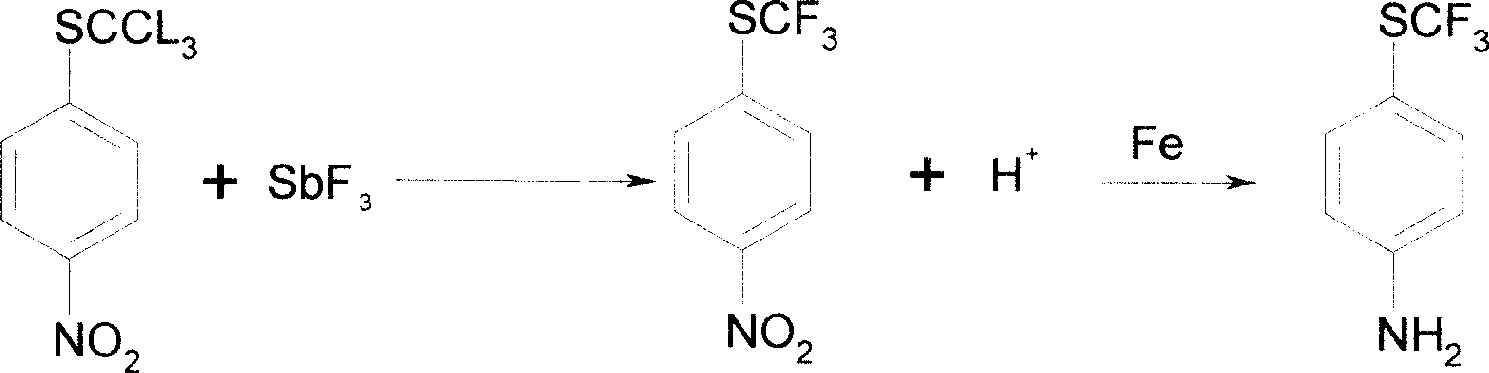
[0043] Reaction 3: Add 52g of antimony trifluoride to 200g of chlorinated substance and react at 40-60℃ to obtain fluoride; 1226g of water, 277g of iron powder, 24g of hydrochloric acid, and 14g of ammonium chloride. -50°C, add fluoride, stir and heat to reflux for 2 hours,...
specific Embodiment approach 2
[0051] Reaction 1: Mix 500 g of methanol and 158 g of p-nitrochlorobenzene until it is fully dissolved, and add dropwise a mixture of sulfur, sodium sulfide and methanol. Keep at 60-65°C for 2 hours, cool down, add 1000g of water, let cool, add 192g of dimethyl sulfate dropwise, and adjust the pH to greater than 9 with sodium hydroxide solution. After the reaction, 154.5 g of methyl sulfide was obtained by spin filtration. The yield was 91.6%.
[0052] Reaction 2: 2000g chloroform and 400g methyl sulfide, stir evenly, control the temperature between 70-75°C, let the chlorine gas react for about 3.5 hours, and filter to obtain 598.3g chloro compound. The yield was 92.3%.
[0053] Reaction 3: Add 52g of antimony trifluoride to 200g of chlorinated substance and react at 40-60℃ to obtain fluoride; 1226g of water, 277g of iron powder, 24g of hydrochloric acid, and 14g of ammonium chloride. -50°C, add fluoride, stir and heat to reflux for 2 hours, perform steam distillation, and steam u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com