Method for preparing methyl nitrite by utilizing reaction composite reinforcement
A technology of methyl nitrite and reaction, which is applied in the field of preparing methyl nitrite by using reaction compound strengthening, can solve the problems of low selectivity of methyl nitrite, high nitric acid content and high energy consumption, and achieves enhanced raw material utilization rate, The effect of reducing nitric acid content and environmentally friendly preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
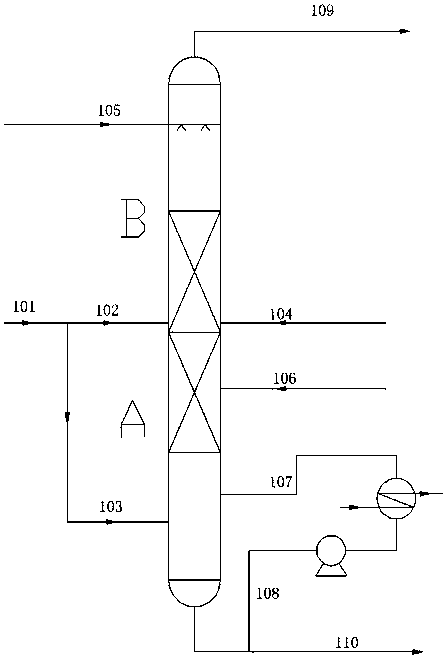
[0024] The nitrogen oxide mixed gas 100NM3 / h is input through the first material pipeline 101, which contains N2 70%, NO 10%, CO 20%, through the flow regulating valve, the distribution ratio of the second material pipeline 102 is 70%, and the third The distribution ratio of material line 103 is 30%. Methanol is input at 12.8Kg / h through the fifth material pipeline 105, oxygen is input at 1.6NM3 / h through the fourth material pipeline 104, nitric acid is not input through the sixth material pipeline 106, and the seventh material pipeline 107 and the eighth material pipeline 108 of the circulating material pipeline are opened , to control the reaction temperature. Finally control the reaction temperature of the esterification reaction B to be 40°C and the pressure to be 0.4Mpa, and control the temperature of the nitric acid reduction reaction A to be 60°C and the pressure to be 0.41Mpa. After the stable reaction, the ninth material pipeline 109 cannot measure the oxygen content....
Embodiment 2 5
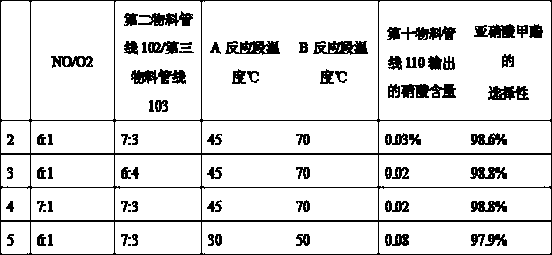
[0026] In this embodiment, the first step is the same as the above embodiment, except that the parameter ratio is changed. The parameters and experimental results are expressed in the form of Table 1.
[0027] Table 1
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com