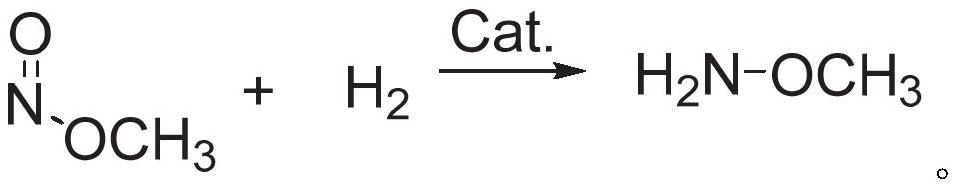
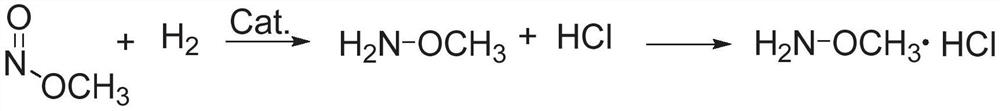Method for preparing methoxyamine, method for preparing methoxyamine hydrochloride
A technology of methoxyamine hydrochloride and methoxyamine, which is applied in the field of coal chemical industry, can solve the problems of high energy consumption for dehydration and separation, consumption of large oil resources, etc., and achieves the requirements of reducing equipment, improving production efficiency, and being easy to industrialized production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
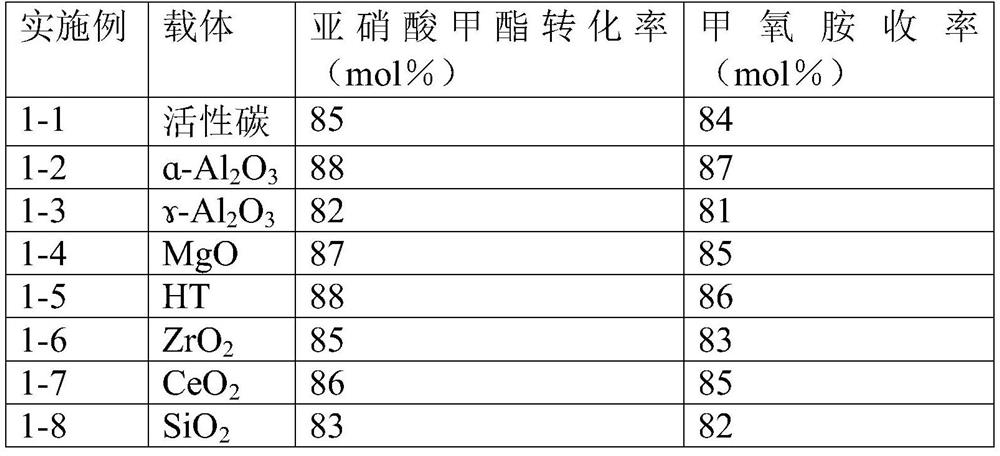
[0056] Embodiment 1 Different nickel-based catalysts catalyze the reaction of hydrogen reduction methyl nitrite to produce methoxyamine
[0057] The nickel-based catalysts of different carriers were tabletted, pulverized, and passed through a 10-mesh sieve, and 5g of nickel-based catalysts (1wt%) with a particle size of less than 10 meshes were packed into a glass tube reactor with a diameter of 15mm. The reaction gas composition was 23.4vol %H 2 , 33.0vol% methyl nitrite, 3.4vol% Ar (internal standard), 40.2vol% N 2 , the reaction temperature is 70°C, and the total WHSV of the reaction gas is 1.0h -1 , the reduction reaction was carried out under normal pressure.
[0058] The results obtained on different supported catalysts are shown in Table 1.
[0059] Table 1 Catalytic hydrogen reduction of methyl nitrite to methoxyamine by different nickel-based catalysts
[0060]
[0061] As can be seen from the results in Table 1, different nickel-based catalysts catalyze the hy...
Embodiment 2
[0062] Embodiment 2 Catalyst particle diameter influence
[0063] 1wt%Ni / ɑ-Al 2 o 3 The nickel-based catalyst was tabletted, crushed, and passed through sieves with different meshes, and 5g of 1wt%Ni / ɑ-Al with a particle size smaller than the corresponding meshes was taken. 2 o 3 Put it into a glass tube reactor with a diameter of 15mm, and the reaction gas composition is 23.4vol%H 2 , 33.0vol% methyl nitrite, 3.4vol% Ar (internal standard), 40.2vol% N 2 , the reaction temperature is 70°C, and the WHSV is 1.0h -1 , the reduction reaction was carried out under normal pressure.
[0064] The results obtained from the reaction results of catalysts with different particle sizes are shown in Table 2.
[0065] Table 2 Catalytic hydrogen reduction of methyl nitrite to methoxyamine by nickel-based catalysts with different particle sizes
[0066]
[0067] It can be seen from the results in Table 2 that the particle size of the catalyst has a significant impact on the conversio...
Embodiment 3
[0068] Embodiment 3 nickel loading influence
[0069] The particle diameter is that 20 mesh 5g nickel-based catalysts are packed into a glass tube reactor with a diameter of 15mm, and the reaction gas composition is 23.4vol%H 2 , 33.0vol% methyl nitrite, 3.4vol% Ar (internal standard), 40.2vol% N 2 , the reaction temperature is 70°C, and the WHSV is 1.0h -1 , the reduction reaction was carried out under normal pressure.
[0070] The reaction results on different catalysts are shown in Table 3.
[0071] Table 3 Ni-based catalysts with different nickel loadings catalyzed hydrogen reduction of methyl nitrite to methoxyamine
[0072]
[0073] It can be seen from the results in Table 3 that the nickel loading has a significant impact on the conversion rate of methyl nitrite. As the nickel loading increases, the conversion rate of methyl nitrite gradually increases and then remains the same. The trend of methoxyamine yield is the same as the conversion rate of methyl nitrite....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com