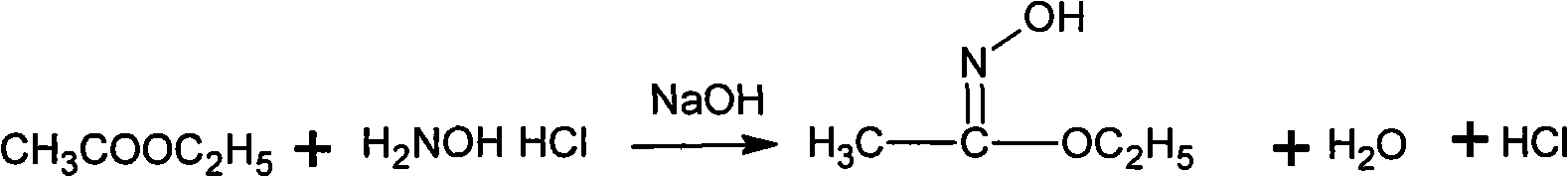
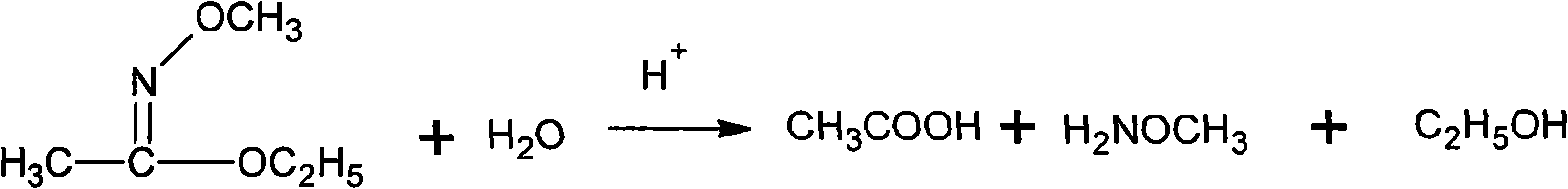
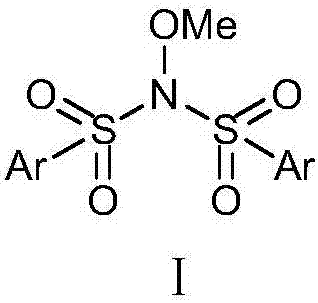
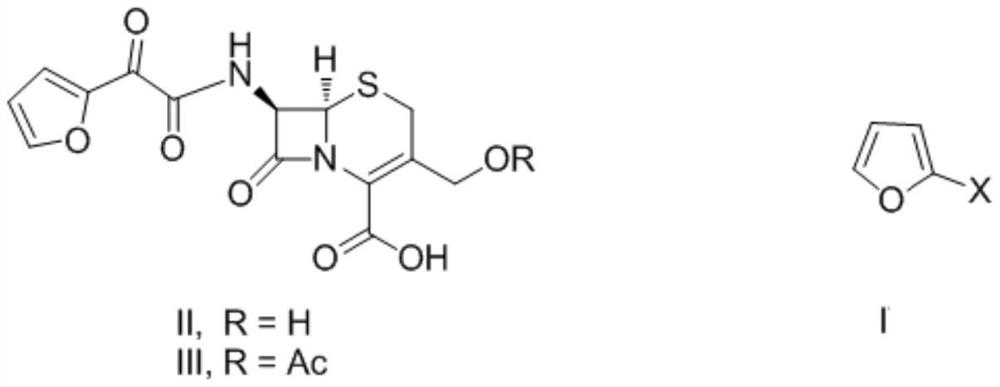
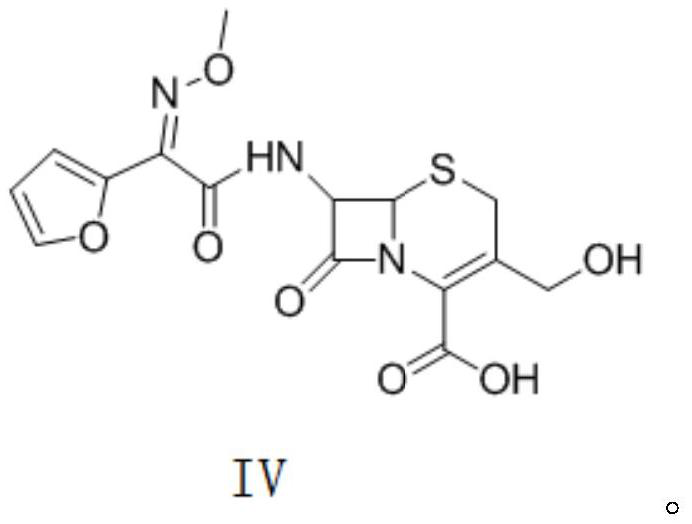
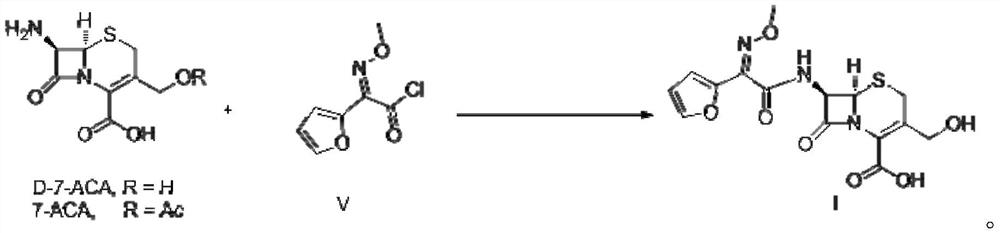
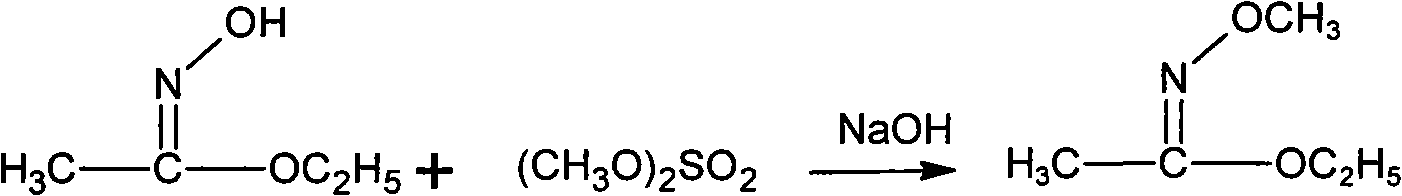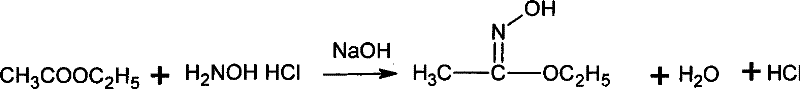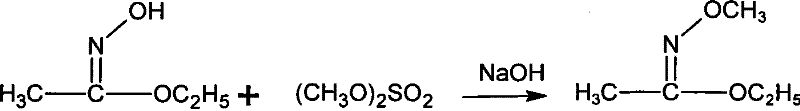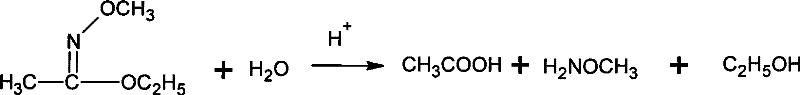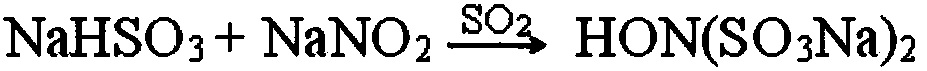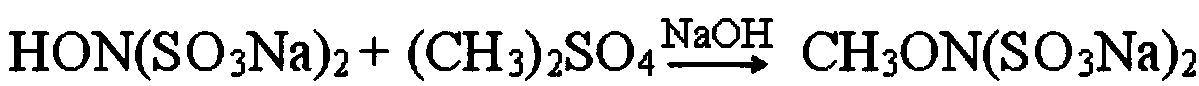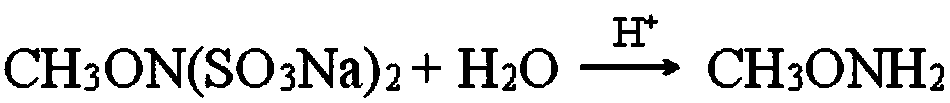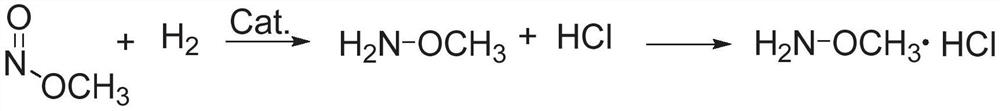Patents
Literature
38 results about "METHOXYAMINE HYDROCHLORIDE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
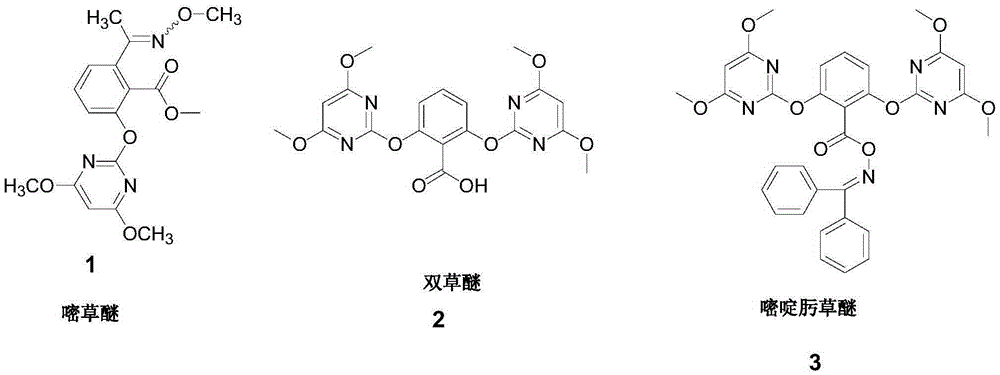
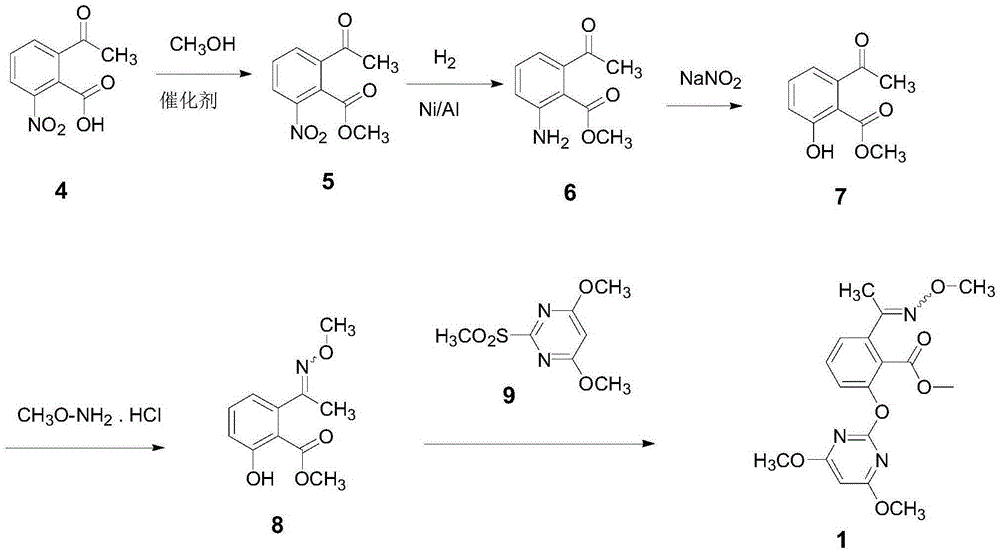
Method for synthesizing methoxamine hydrochloride
ActiveCN101357895AReduce usageImprove the operating environmentOrganic chemistryHalohydrocarbonHydroxylamine Hydrochloride
The invention provides a method for synthesizing methoxyamine hydrochloride and includes the following steps: ethyl acetate and hydroxylamine hydrochloride are added into a reaction vessel and 10 to 30 percent of sodium hydroxide solution is instilled for oximation reaction; then dimethyl sulfate is instilled, and at the same time sodium hydroxide solution with the mass fractions of 10 to 30 percent is instilled for methylation reaction; cold water is added after the temperature is decreased and a halohydrocarbon solvent is adopted for extraction; pressure is reduced under the temperature of 30 to 50 DEG C to recover the halohydrocarbon solvent and the product obtained is added into inorganic acid solution for hydrolysis reaction; after the hydrolysis is over, hydrochloride is used to obtain the product methoxyamine hydrochloride. The synthetic method which is simple improves the operation environment and increases the yield ratio.
Owner:JIANGSU QINGQUAN CHEM CO LTD
Method for synthesizing methoxamine hydrochloride
The invention provides a method for synthesizing methoxyamine hydrochloride and includes the following steps: ethyl acetate and hydroxylamine hydrochloride are added into a reaction vessel and 10 to 30 percent of sodium hydroxide solution is instilled for oximation reaction; then dimethyl sulfate is instilled, and at the same time sodium hydroxide solution with the mass fractions of 10 to 30 percent is instilled for methylation reaction; cold water is added after the temperature is decreased and a halohydrocarbon solvent is adopted for extraction; pressure is reduced under the temperature of 30 to 50 DEG C to recover the halohydrocarbon solvent and the product obtained is added into inorganic acid solution for hydrolysis reaction; after the hydrolysis is over, hydrochloride is used to obtain the product methoxyamine hydrochloride. The synthetic method which is simple improves the operation environment and increases the yield ratio.
Owner:JIANGSU QINGQUAN CHEM CO LTD
Chemical derivation-based detection method for hemolymph metabolin of migratory locust
InactiveCN102866220AImprove extraction efficiencyGood reproducibilityComponent separationWater bathsGas phase
The invention discloses a chemical derivation-based detection method for hemolymph metabolin of migratory locust, and relates to the field of animal ecology and analytical chemistry. The detection method specifically comprises the following steps of: taking hemolymph, deproteinizing by adding ethanol-acetonitrile mixed solvent, supersonically extracting, and centrifuging at a high speed to obtain metabolin extracting solution; drying the extracting solution by means of pressure reduction, adding methoxamine hydrochloride-pyridine solution, supersonically dissolving, blending by means of vortex, and deriving in an oximation way in a constant-temperature water bath; adding N-methyl-N-trimethylsilane-trifluoroacetamide reagent, blending by means of vortex, and deriving in an alkylation way in the constant-temperature water bath; and adding methyl oleate-normal heptane solution to compensate the volume, blending by means of vortex, and analyzing by a spare gas chromatography-mass spectra coupling technique. The detection method provided by the invention is convenient, high-efficiency, mild in reaction conditions, good in repeatability, wide in metabolin coverage, suitable for developing the multi-center and large-sample research, and capable of providing help for knowing the biological law of various migratory locust variations and the occurrence mechanism of the locust plague.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Glycosylated derivatives of bufolin and its preparation method and application in the preparation of antitumor drugs
ActiveCN103288911BSmall toxicityImprove anti-tumor activitySteroidsAntineoplastic agentsSolubilityKetone
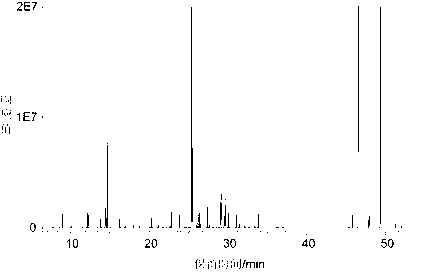
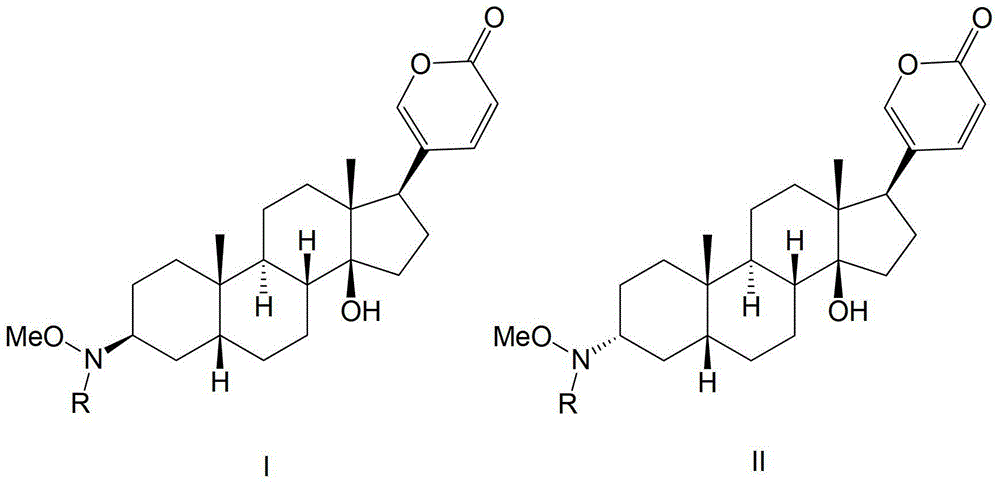
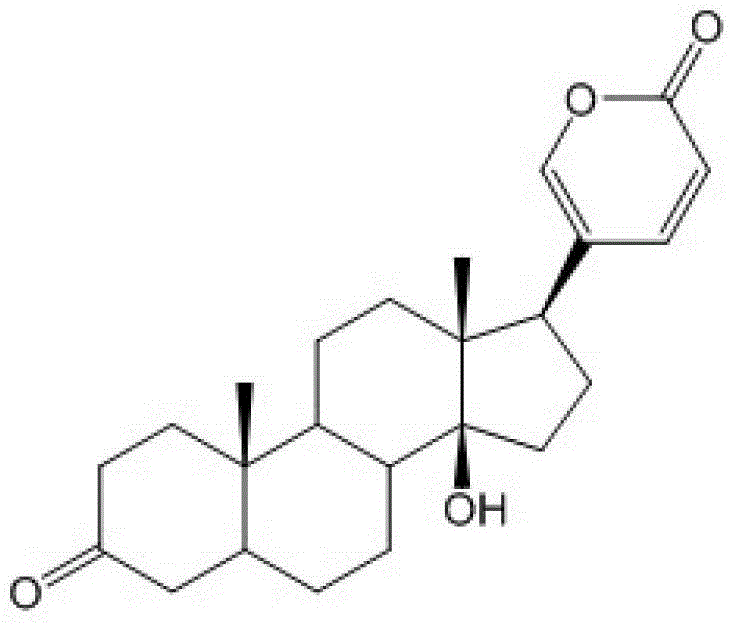
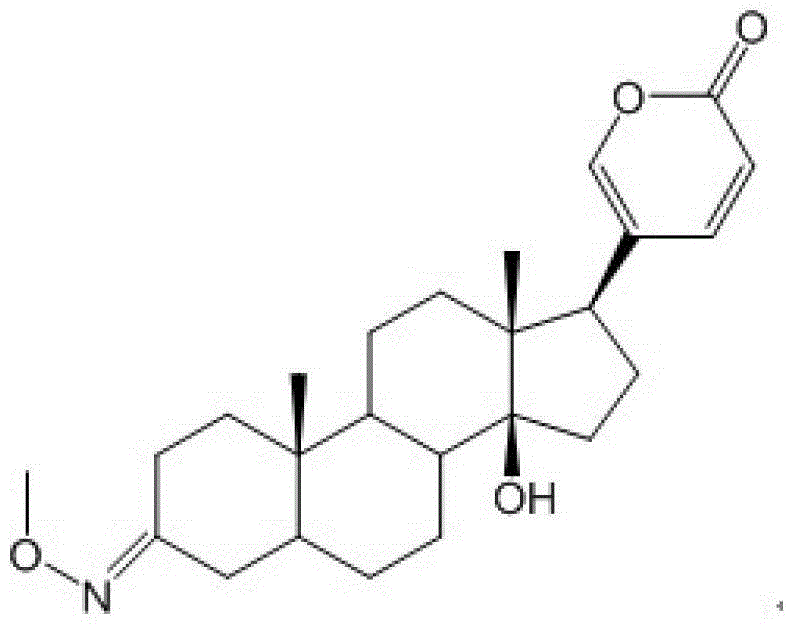
The invention discloses a bufalin glycosylation derivative and a preparation method and application thereof in preparation of anti-tumour medicaments. The structure of the bufalin glycosylation derivative is shown in a formula I or formula II. The synthesis method of the bufalin glycosylation derivative comprises the following steps of: oxidizing bufalin into a ketone-form derivative by pyridinium chloropyridine hydrochloride; then performing a nucleophilic addition reaction with methoxyamine hydrochloride; removing monomolecular water to generate an oxime-form intermediate; enabling the oxime-form intermediate to react with a tert-butylamine borane hydrochloride compound to generate alpha-configuration aglycone and beta-configuration aglycone; and finally enabling the aglycones of two configurations to react with reducing sugar respectively to generate the bufalin glycosylation derivative. According to the method disclosed by the invention, bufalin is modified to be the glycosylation derivative thereof, and the water solubility can be improved while the anti-tumour activity is kept and enhanced; and moreover, in-vivo experiments prove that the toxic and side effects of the bufalin glycosylation derivative disclosed by the invention on normal cells are reduced compared with those of a parent compound of bufalin.
Owner:GUANGXI WUZHOU PHARMA GRP
Synthetic method of kresoxim methyl
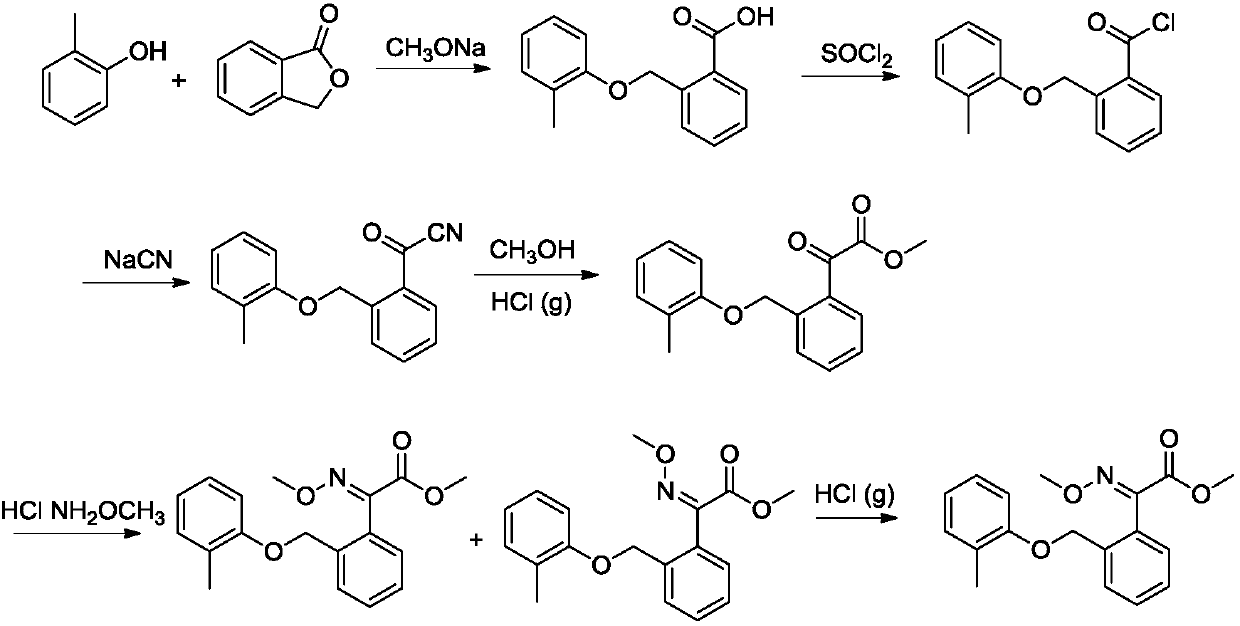
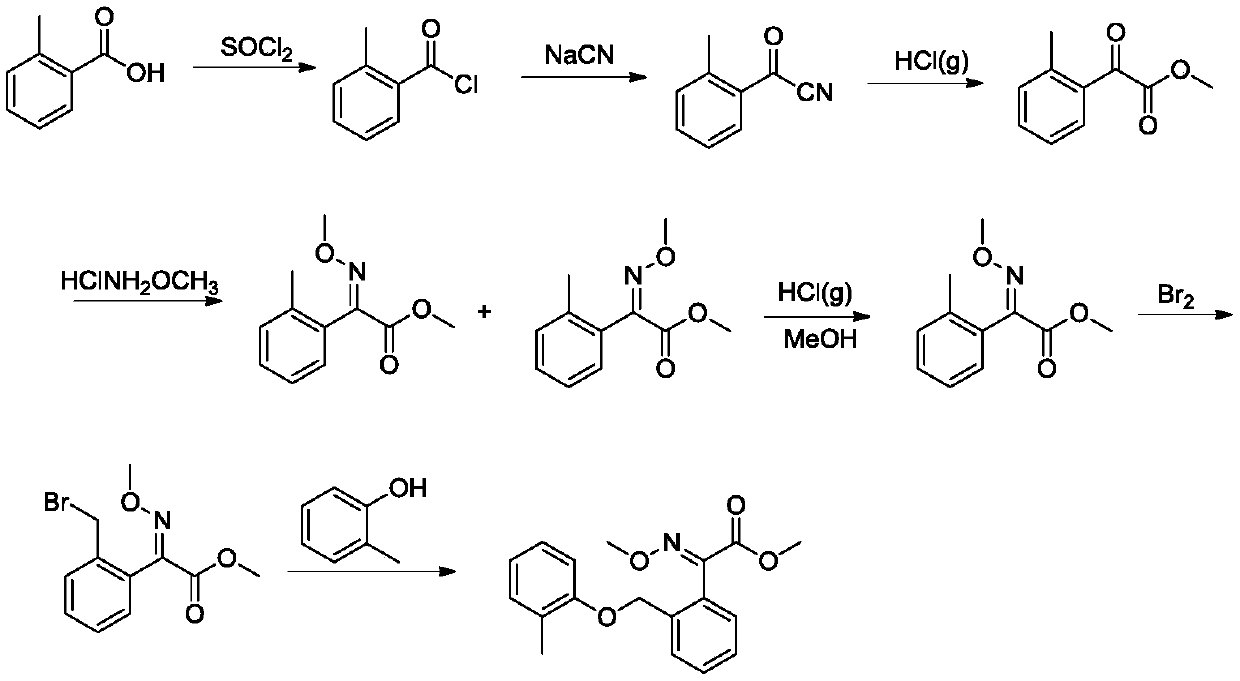
The invention belongs to the technical field of fine chemical industry, and relates to a pesticide chemical synthesis technology, especially to a synthetic method of kresoxim methyl. The synthetic method uses 2-(2-methylphenoxymethyl)benzoyl cyanide as an initial raw material to react with methoxyamine hydrochloride. Hydrochloric acid gas is introduced into a system after the reaction is completed. The method has the advantages of short reaction period, high conversion rate and excellent product quality. The content of the synthesized kresoxim methyl is above 99%, which satisfies the technicalrequirements of the market for kresoxim methyl.
Owner:JINGBO AGROCHEM TECH CO LTD
Method for screening almond-flavored tea tree germplasm resources
The invention discloses a method for screening almond-flavored tea tree germplasm resources. The method comprises the following steps of selecting three parts of tea raw materials, wherein the two parts have the almond-flavored tea tree germplasm resources, and the rest part does not have the almond-flavored tea tree germplasm resources; weighing 50-70 mg of a sample, putting the sample into a grinding machine for grinding, taking out the sample, and carrying out ultrasonic extraction for 20-40 minutes; adding 100-300 [mu]L of chloroform into the sample, performing uniform mixing, adding water, performing uniform mixing, and carrying out ultrasonic extraction for 10-30 minutes; adding a methoxyamine hydrochloride pyridine solution into a glass-derived small bottle, carrying out vortex oscillation for 2 minutes, carrying out oximation reaction in a concussion incubator, taking out the sample, and placing the sample at the room temperature for 30 minutes; and collecting sample data to perform GC-MS metabonomics analysis. Through the technical scheme, the content of mandelonitrile in tea leaf metabolites can be rapidly analyzed, so that the screening work of the almond-flavored tea tree germplasm resources can be rapidly realized, and the working time of original 4-year breeding is greatly shortened.
Owner:TEA RES INST GUANGDONG ACAD OF AGRI SCI
Preparation method of paddy field herbicide pyriminobac-methyl
InactiveCN105272925ASuitable for mass productionHigh yieldOrganic chemistryAcetylsalicylic acid methyl esterNitrobenzene
The invention belongs to the field of agricultural chemistry, and particularly relates to a preparation method of a safe and efficient broad-spectrum paddy field herbicide pyriminobac-methyl ((E)-pyriminobac-methyl). The method comprises the following steps: with 2-acetyl-6-nitrobenzoic acid as a raw material, carrying out esterification reaction under an acid catalyst to prepare the 2-acetyl-6-nitrobenzene methyl formate; carrying out hydrogenation reduction reaction under catalysis of Ni / Al to prepare 2-amino-6-acetylbenzene methyl formate; carrying out diazotization to prepare the 6-methyl acetylsalicylate; carrying out imidization on methoxyamine hydrochloride under basic catalysis to prepare a salicylic acid midbody 6-(1-(N-methoxy)ethyl) methyl salicylate; and finally carrying out condensation reaction on the 2-mesyl-4,6-dimethoxy pyrimidine to prepare the pyriminobac-methyl. The method is a clean production technique and is suitable for mass production.
Owner:CHANGZHOU UNIV
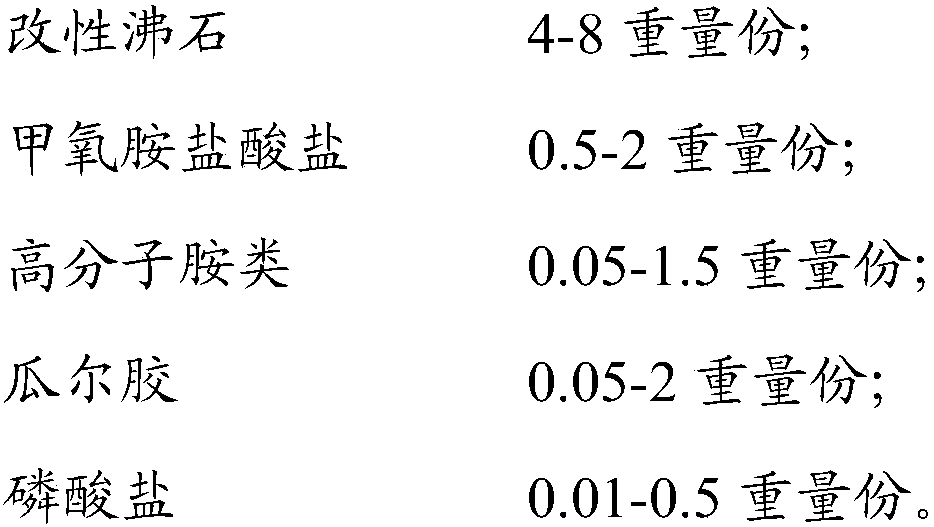
Aldehyde removing gypsum board and preparation method thereof
ActiveCN109928704ALarge adsorption capacityEnhanced adsorptionDispersed particle separationCeramicwarePhosphateGuar gum
The invention provides an aldehyde removing gypsum board and a preparation method thereof, wherein the aldehyde removing gypsum board comprises a mixture of modified zeolite, methoxyamine hydrochloride, polymer amine, guar gum and phosphate. According to the present invention, the aldehyde removing gypsum board can quickly capture indoor formaldehyde and decompose, such that the indoor formaldehyde concentration can be effectively reduced, and the aldehyde removing effect can be maintained for a long time; and the aldehyde removing gypsum board has excellent mechanical property.
Owner:BEIJING NEW BUILDING MATERIALS PLC
Method for co-producing methoxyamine hydrochloride and n,o-dimethylhydroxylamine hydrochloride
ActiveCN105859575BSuitable for industrial productionThe process is simple and reliableOrganic chemistryMethylating AgentHydroxylamine
The invention relates to the technical field of compound synthesis methods, particularly a method for coproducing vasoxine hydrochloride and N,O-dimethylhydroxylamine hydrochloride. The method comprises the following steps: carrying out methylation reaction on hydroxylamine salt under alkaline conditions by using a methylating agent to obtain a reaction solution containing vasoxine and N,O-dimethylhydroxylamine, rectifying to separate a vasoxine bottom solution and an N,O-dimethylhydroxylamine crude distillate, respectively adding hydrochloric acid for salification, concentrating and crystallizing under reduced pressure, cooling, carrying out vacuum filtration, recrystallizing with water or methanol, and drying to obtain the vasoxine hydrochloride product and N,O-dimethylhydroxylamine hydrochloride product. The method has the advantages of simple and reliable technique, high product quality, high total yield and low comprehensive cost, and is more friendly to the environment and suitable for industrial production.
Owner:宁波四明化工有限公司
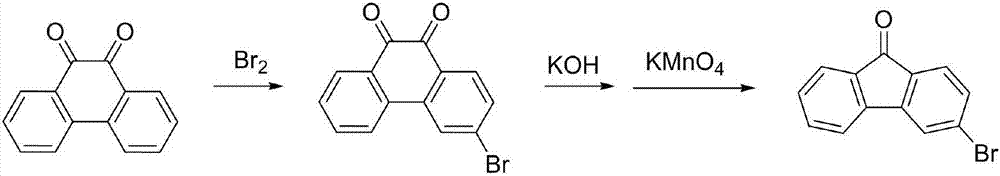
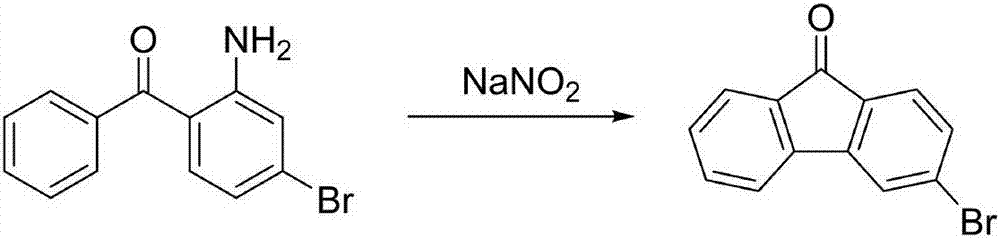
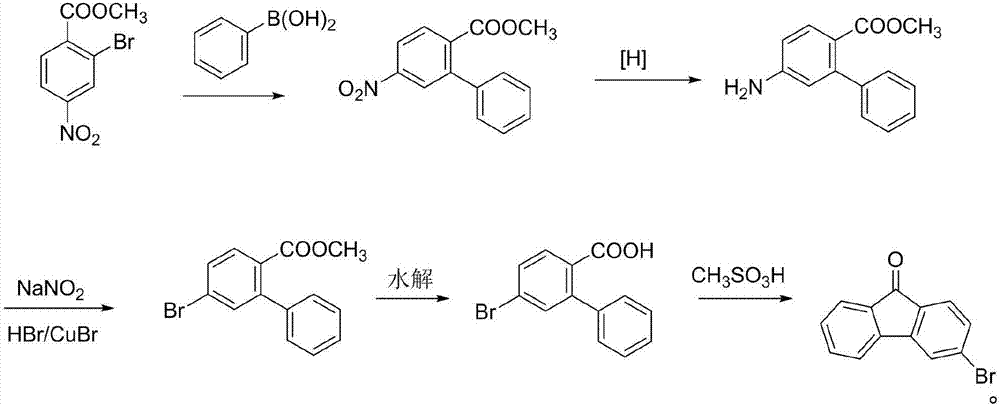
3-bromofluorenone preparation method
ActiveCN107056595ARaw materials are cheap and easy to getMild reaction conditionsOximes preparationCarbonyl compound preparation by hydrolysisBromineFluorenone
The invention belongs to the technical field of fluorine compound preparation, in particular to a 3-bromofluorenone preparation method. The 3-bromofluorenone preparation method comprises the following steps: A, oximation reaction: adding fluorenone and methoxamine hydrochloride into a solvent, performing oximation reaction, and performing reaction quenching to obtain fluorenone methoxy oxime; B, bromination reaction: adding a catalyst, bromine and the fluorenone methoxy oxime obtained in the step A into the solvent, and performing reaction quenching to obtain a bromide product; C, hydrolysis reaction: adding the bromide product obtained in the step B into the solvent, and performing hydrolysis reaction at the temperature of 50 to 100 DEG C to obtain 3-bromofluorenone. The preparation method is simple and low in cost, the obtained 3-bromofluorenone is high in yield and high in purity, the reaction conditions are mild, and the 3-bromofluorenone is suitable for industrial production.
Owner:VALIANT CO LTD
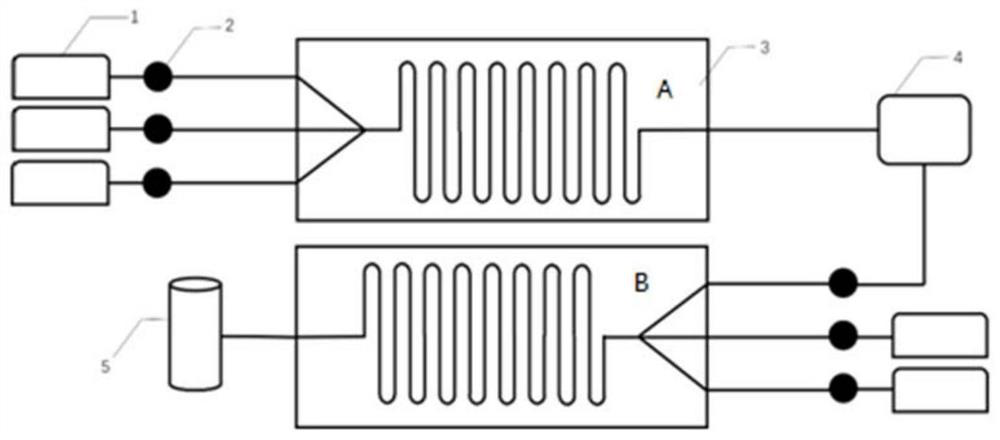
Method for preparing methoxyamine hydrochloride by adopting microreactor
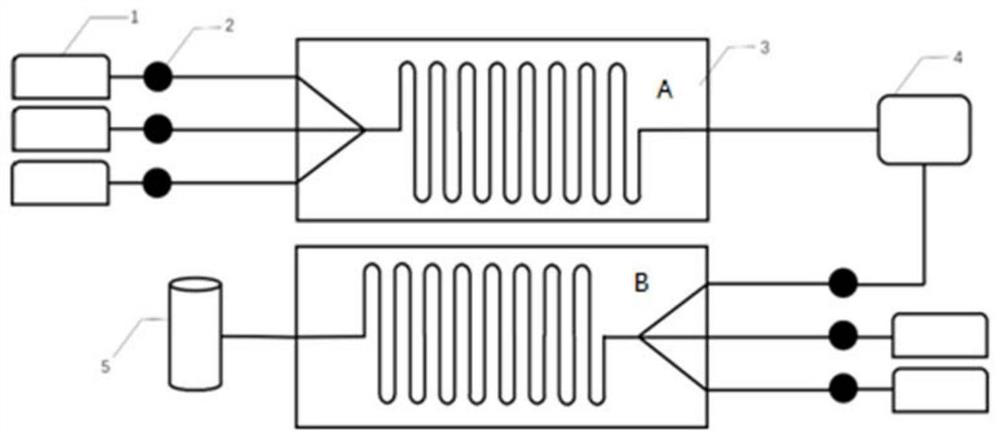
ActiveCN113045451AAccurate control precisionReact SafeOrganic chemistryChemical/physical/physico-chemical microreactorsHydroxylamineDistillation
The invention belongs to the field of synthesis of fine chemical intermediates, and discloses a method for preparing methoxyamine hydrochloride by adopting a microreactor. The method specifically comprises the steps of preparation of acetohydroxylamine, preparation of acetomethoxylamine and preparation of methoxylamine hydrochloride, wherein the preparation processes of acetohydroxylamine and acetomethoxylamine are carried out in a microreactor. Specifically, a hydroxylamine hydrochloride solution, ethyl acetate and alkali liquor are pumped into a micro-channel reaction module A and mixed in a micro-channel, then the mixture, dimethyl sulfate and alkali liquor are pumped into a micro-channel reaction module B again, collection is conducted after mixed reaction, and then the methoxylamine hydrochloride is obtained through the processes of hydrolysis, distillation, neutralization, salification, concentration and the like. Compared with the prior art, the method has the advantages that the yield of methoxyamine hydrochloride is effectively improved by utilizing the microreactor, the generation of three wastes is reduced, the reaction time is shortened, and the production cost is reduced.
Owner:南京科力硕生物科技有限公司
Method for synthesizing methoxyamine hydrochloride
PendingCN113754557AEmission reductionMild reaction conditionsProcess control/regulationOrganic chemistryPtru catalystSodium hydroxide
The invention relates to the technical field of compound synthesis methods, and discloses a method for synthesizing methoxyamine hydrochloride. The method comprises the following steps: adding water, sodium hydroxide, diacetylmonoxime and a phase transfer catalyst into a reaction kettle; after cooling, adding a methylation reagent; conducting standing for layering to obtain an organic layer, a water layer and a distilled water layer, collecting distillate, combining an oil layer and the distillate, and adding hydrochloric acid for mixing to obtain a mixed solution; feeding and rectifying the mixed solution from the middle of a rectifying column, recovering butanone and methanol at the top of the column, recovering a methoxyamine hydrochloride solution at the bottom of the column, and carrying out evaporating and dehydrating to obtain a concentrated solution; and cleaning a reaction kettle, wherein a stirring mechanism and a cleaning mechanism are arranged in the reaction kettle, the cleaning mechanism comprises a scraping plate, a spraying pipe, a lifting assembly and a rotating assembly, a water inlet pipe and a cleaning nozzle are connected to the spraying pipe, the reaction kettle comprises a kettle body and a kettle cover, the water outlet end of the cleaning nozzle faces the side wall of the kettle body, and the scraping plate is attached to the side wall of the kettle body. The method has the effect of conveniently cleaning residues on the side wall of the kettle body.
Owner:宁波四明化工有限公司
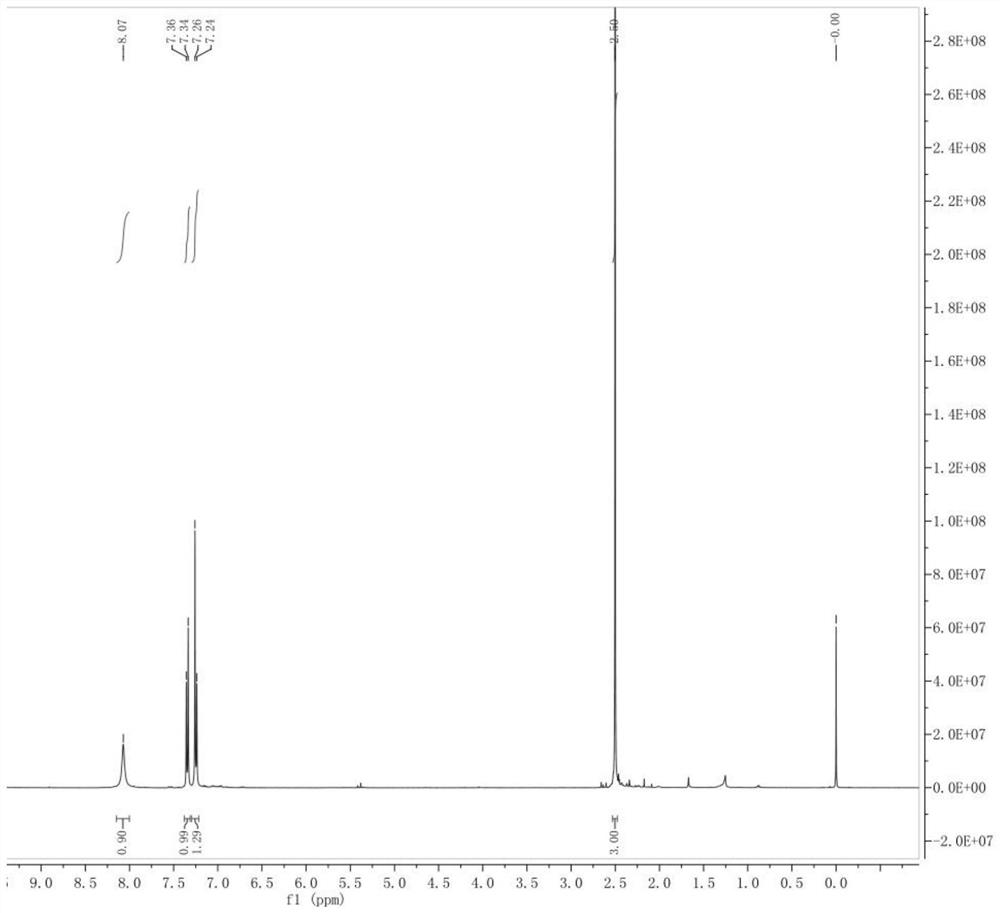
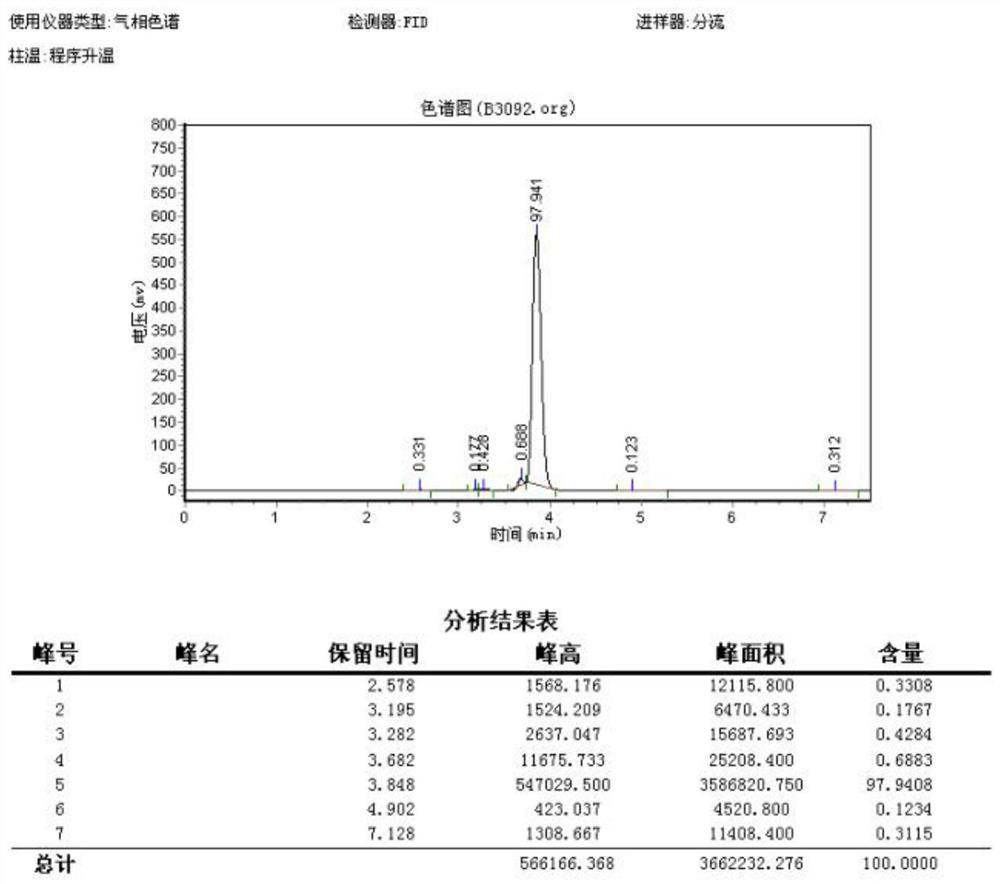
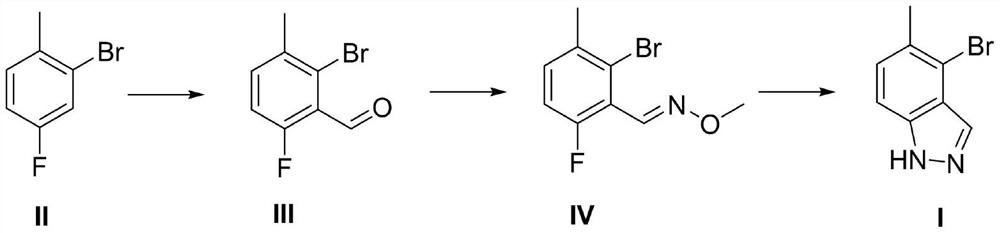
Preparation method of 4-bromo-5-methyl-1H-indazole
InactiveCN112321510AReduce pollutionEasy to handleOrganic chemistryLithium diisopropylamidePotassium carbonate
The invention discloses a preparation method of 4-bromo-5-methyl-1H-indazole, wherein the preparation method comprises the specific steps: (1) reacting a compound (II) in the presence of lithium diisopropylamide to generate a lithium reagent, and reacting the lithium reagent with dimethylformamide to generate a compound (III); (2) reacting the compound (III) with methoxyamine hydrochloride and potassium carbonate to obtain a compound (IV); and (3) cyclizing the compound (IV) under the participation of hydrazine hydrate to generate a compound (I). The preparation method is higher in yield.
Owner:无锡双启科技有限公司
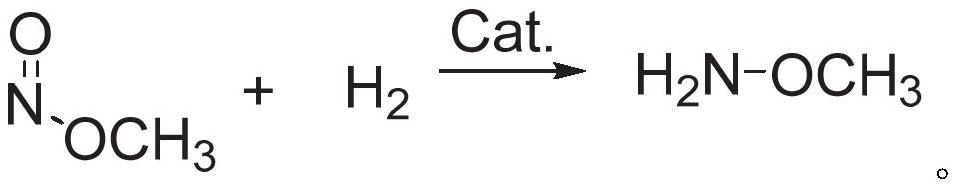
Method for preparing methoxyamine, method for preparing methoxyamine hydrochloride
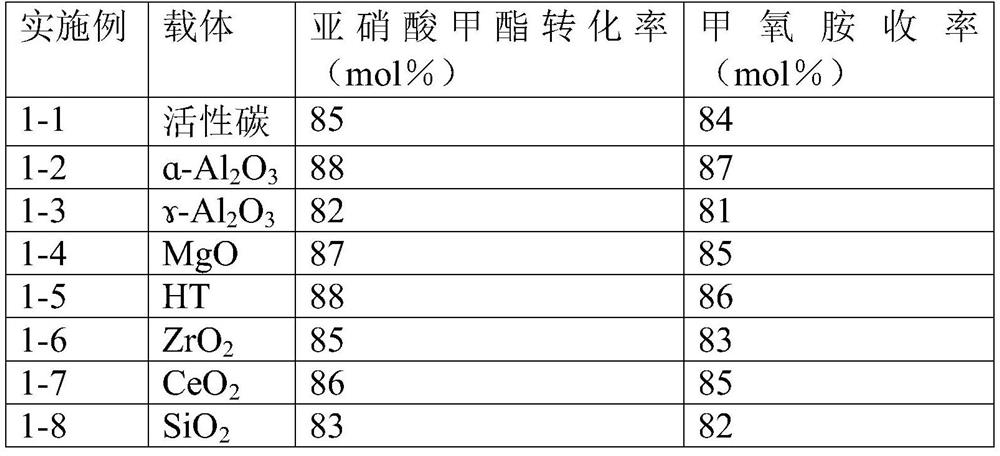
ActiveCN112125822BImprove conversion efficiencyEasy to operateOrganic chemistryPtru catalystMethyl nitrate
The present application discloses a method for preparing methoxyamine, which at least includes: contacting a raw material gas containing methyl nitrite and a reducing agent with a reduction reaction catalyst in a reactor to perform a reduction reaction to obtain methoxyamine. The method can fully utilize the important intermediate methyl nitrite in the coal-to-ethylene glycol process, and the conversion rate of the methyl nitrite is high. The present application also provides a preparation method of methoxyamine hydrochloride obtained by the above method as raw material.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Refining method of methoxyamine hydrochloride
ActiveCN113185426AHigh purityDoes not affect continuityMembranesOrganic chemistryInorganic saltsChemical industry
The invention relates to the field of chemical industry, in particular to a refining method of methoxyamine hydrochloride. The refining method comprises the steps of rectifying and removing volatile organic matters and reaction raw materials in reaction liquid by using a rectification method, concentrating by using a concentration tower, adding an organic solvent to separate out inorganic salt, and removing the inorganic salt in kettle liquid by filtering. The hydrophilic polysulfone microporous filtration membrane used in the method has good filtration effect and filtration efficiency, and does not affect the continuity of the whole process; according to the principle that methoxyamine hydrochloride and inorganic salt have different solubility in an organic solvent, the inorganic salt is separated out by adding the organic solvent, and then the purpose of removing the inorganic salt is achieved; and the organic solvent can be recycled, the material cost of production is not increased, the treatment capacity of three wastes is not increased, the methoxyamine hydrochloride obtained through purification by the method has higher purity, and the product quality and the market competitiveness are improved.
Owner:ZHEJIANG JINHUA NEW MATERIALS
Preparation method of Iguratimod intermediate
PendingCN114539104AEasy to getPromote environmental protectionOrganic compound preparationSulfonic acid amide preparationP-nitroanisolePtru catalyst
The invention discloses a preparation method of an Iguratimod intermediate, which comprises the following steps: by taking p-nitroanisole as a raw material, carrying out substitution nucleophilic substitution (VNS) on p-nitroanisole and methoxyamine hydrochloride in the presence of a copper salt catalyst and an acid-binding agent to generate 5-methoxy-2-nitroaniline (compound II); the synthesis method comprises the following steps: carrying out a nucleophilic substitution reaction on 5-methoxy-2-nitroaniline (compound II) and methanesulfonyl chloride to generate a compound III, etherifying the compound III and phenol under the catalysis of a copper salt to generate N-(5-methoxy-2-phenoxy phenyl) methane sulfonamide (compound IV), and reagents used in the synthesis process are non-highly toxic products and are easy to obtain; no iron powder is used in the reaction process, so that iron mud which is harmful to the environment is not generated, and the environmental protection property is high; the reaction operation difficulty is small, the safety is high, and a foundation is laid for industrial preparation of Iguratimod drugs.
Owner:常州佳德医药科技有限公司
Tobacco essence characteristic compound analysis method and application thereof
PendingCN114839285AEffective for oximationFully silanizedComponent separationHydroxylamineInternal standard
The invention provides a tobacco flavor characteristic compound analysis method and application thereof. The tobacco flavor characteristic compound analysis method comprises the following steps: mixing a to-be-detected sample with an internal standard substance, blow-drying, carrying out a mixed reaction with an oximation reagent, carrying out a mixed reaction with a silanization reagent, and finally carrying out GC-MS detection to obtain a result, the oximation reagent is a combination of methoxyamine hydrochloride and hydroxylamine hydrochloride. The analysis method provided by the invention can be used for efficiently and quickly detecting the characteristic compounds in the tobacco essence, and is accurate in detection result and high in precision.
Owner:CHINA TOBACCO JIANGSU INDAL
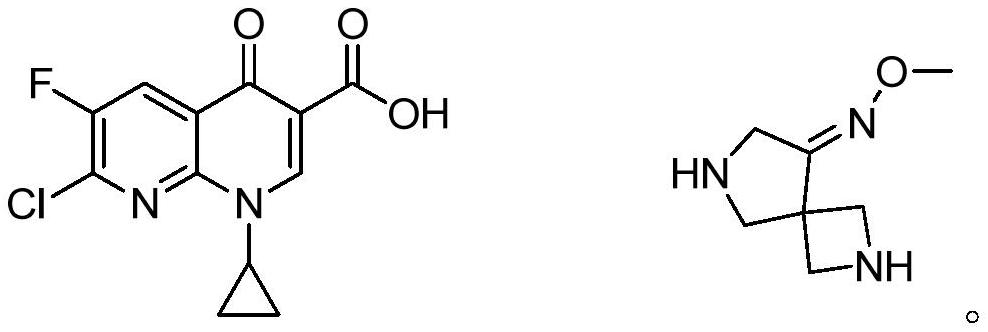
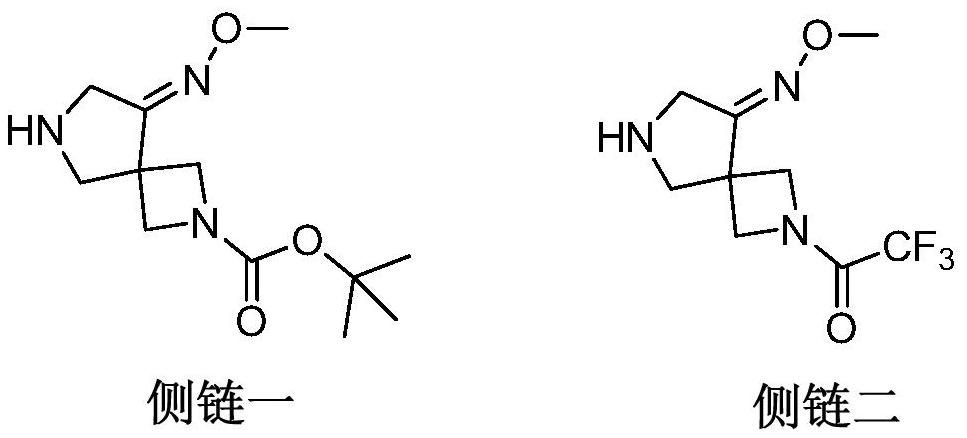
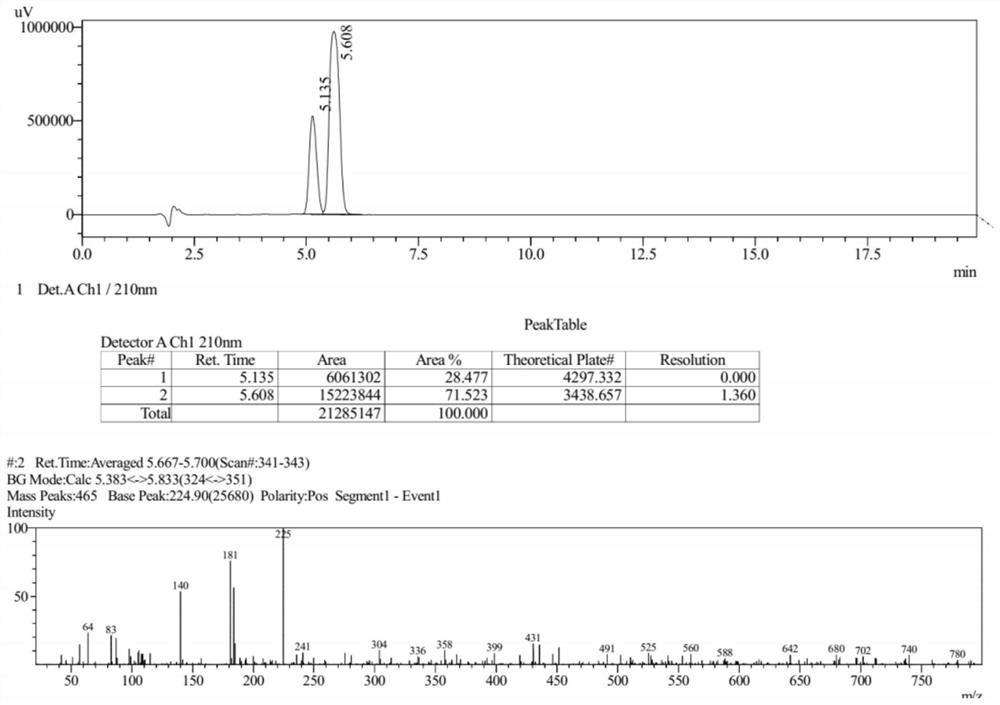
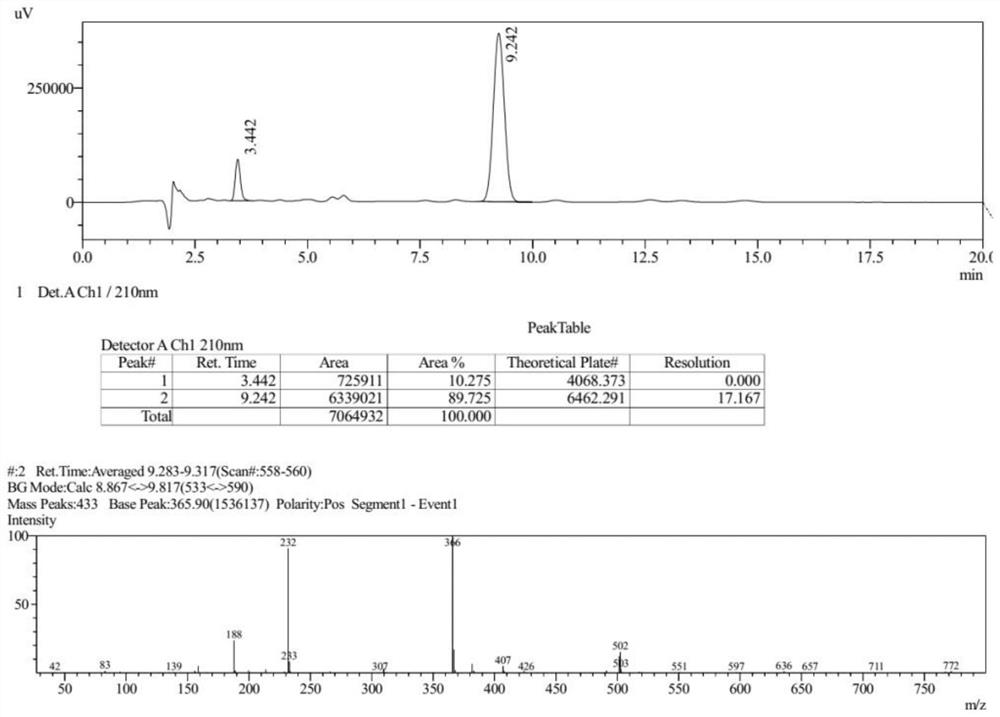
Preparation method of gemifloxacin side chain compound
ActiveCN113773240BSimple processMild reaction conditionsOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupSide chain
The invention discloses a preparation method of gemifloxacin side chain compound. The preparation method comprises the following steps: S1, 1-N-tert-butoxycarbonyl-4-cyano-3-pyrrolidone reacts with methoxylamine hydrochloride to obtain the compound shown in formula (V); S2, in an inert In the atmosphere, at a temperature of 10-50°C and in the presence of palladium carbon, the compound shown in formula (Ⅴ) is subjected to a hydrogenation reduction reaction for 1-3 hours under a hydrogen atmosphere of 0.1-0.5Mpa; then Boc anhydride is added, and the Continue the hydrogenation reduction reaction at 10-50°C and 0.1-0.5Mpa for 4-8 hours to obtain the compound shown in formula (VI); S3, the compound shown in formula (VI) reacts with an acid to remove the protecting group to obtain Gemifloxacin side chain compound represented by formula (I). The preparation method of the present invention has simple process, very mild reaction conditions, and is easy to realize; (2) the raw material price is low, and the cost is low; (3) a new and effective synthesis process route of gemifloxacin side chain is created.
Owner:北京阳光诺和药物研究股份有限公司
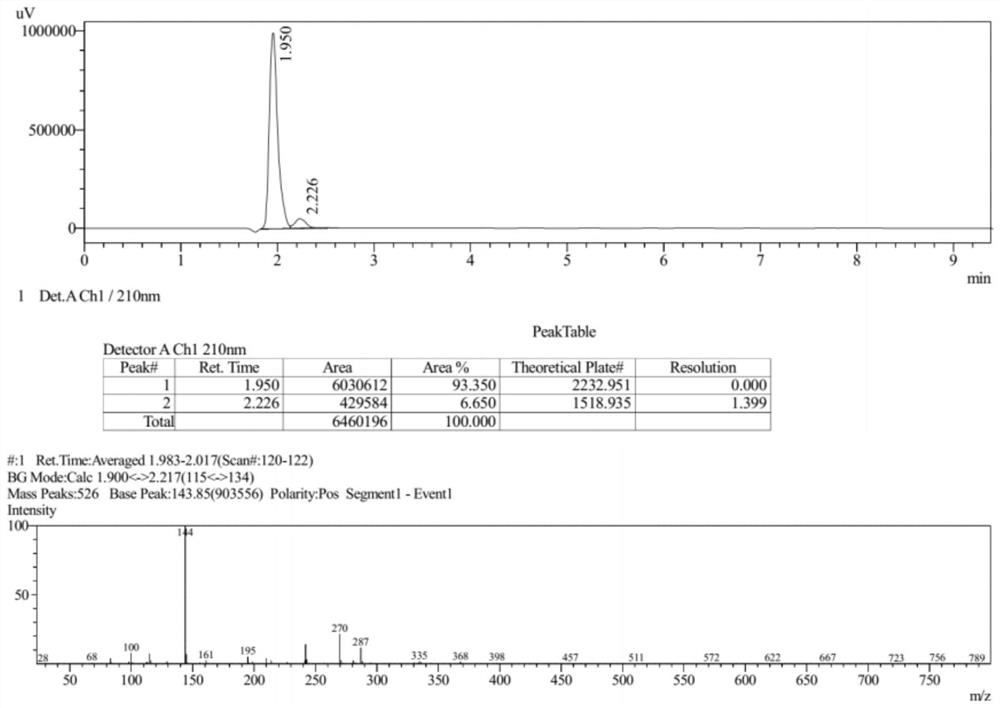
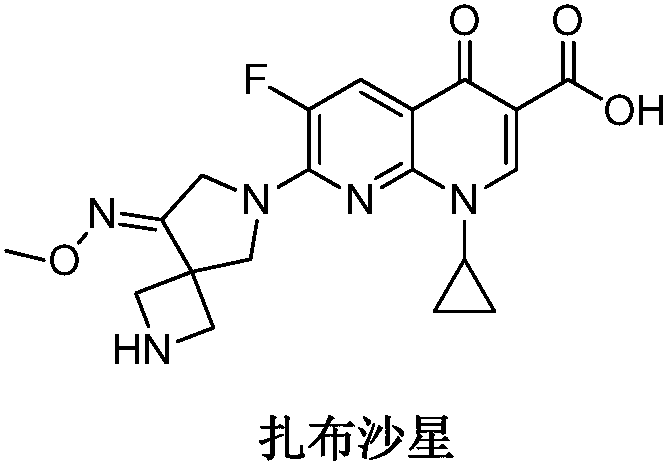
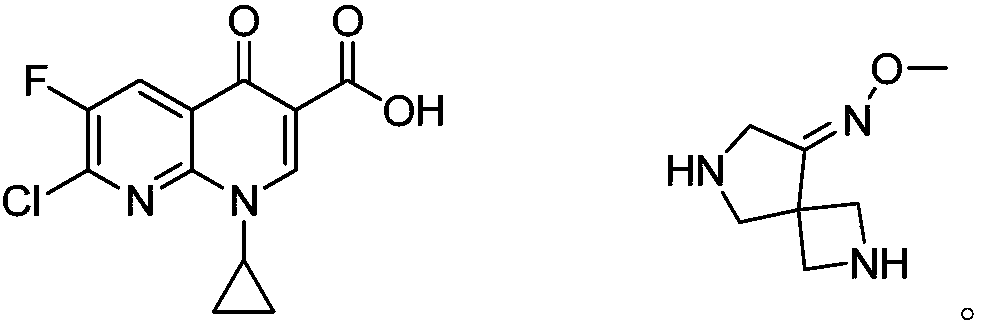
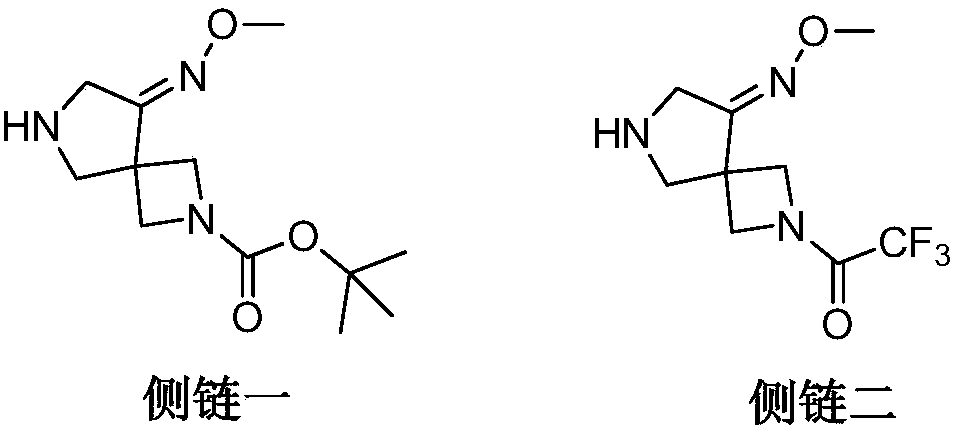
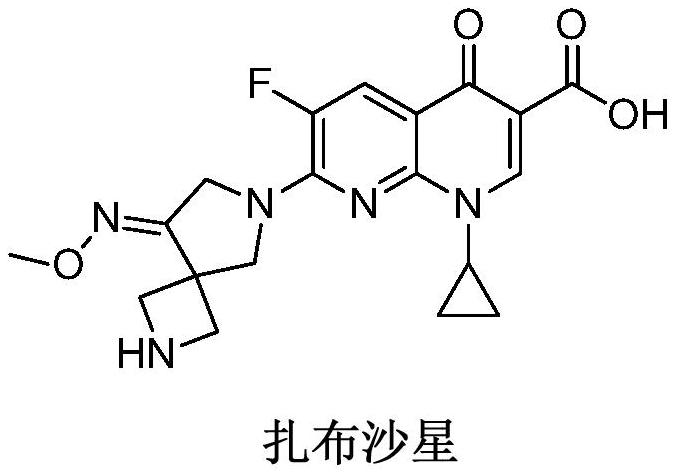
Preparation method of zabofloxacin intermediate
The invention provides a preparation method of a zabofloxacin intermediate. The method comprises: reacting ethyl glycinate hydrochloride with acrylonitrile under catalysis of a base to form an intermediate I; then reacting the intermediate I with Boc anhydride under the function of a strong base to generate an intermediate III; reacting the intermediate III, the methoxyamine hydrochloride and formaldehyde to generate an intermediate IV; and then preparing the zabofloxacin intermediate by reacting the intermediate IV with methanesulfonyl chloride, performing reduction with sodium borohydride, and other steps. The zabofloxacin intermediate is prepared through nine steps with a high yield and high quality by the method, the material adding amount reaches the level of hundreds of grams, and the method is suitable for industrial production.
Owner:沈阳林特制药有限公司
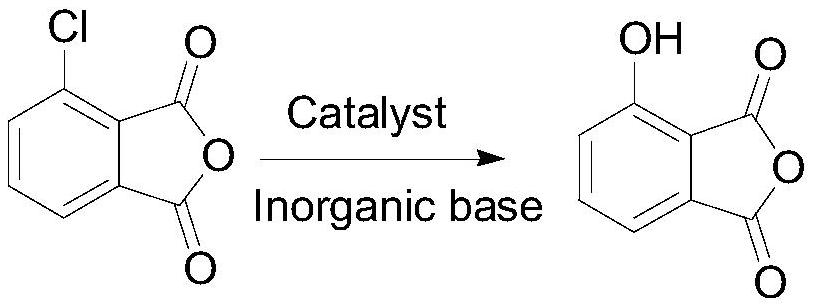
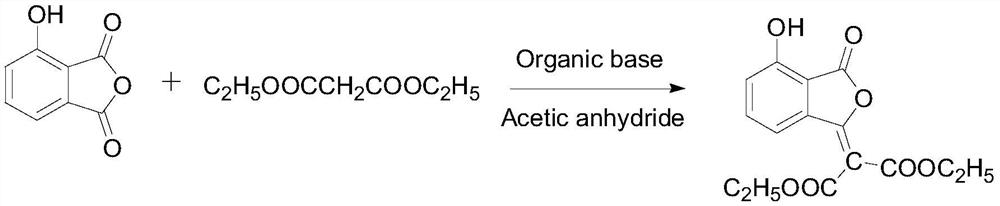
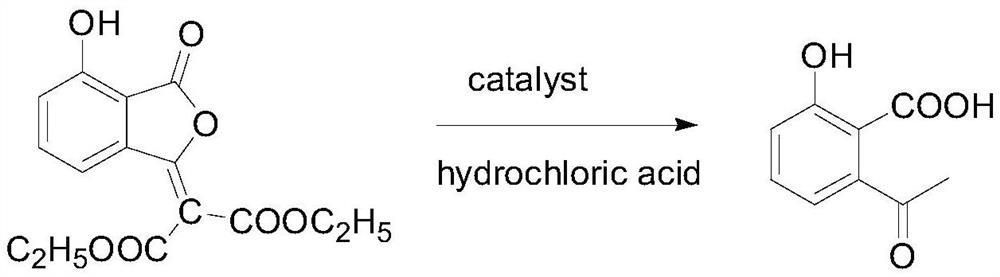
Method for synthesizing herbicide pyriminobac-methyl in paddy field
The invention belongs to the field of fine chemical engineering, and particularly relates to a preparation method of herbicide pyriminobac-methyl for a paddy fields. The preparation method comprises the following steps: synthesizing 3-hydroxy phthalic anhydride by using 3-chlorophthalic anhydride as a new raw material, protecting carbonyl by using diethyl malonate, hydrolyzing to obtain 2-acetyl-6-hydroxy benzoic acid, and esterifying to obtain 2-acetyl-6-hydroxy methyl benzoate; then carrying out imidization reaction with methoxyamine hydrochloride to obtain 2-hydroxy-6-(1-methoxy iminoethyl-methyl)-benzoate, and finally, condensing with 2-tosyl-4, 6-dimethoxypyrimidine to obtain pyriminobac-methyl. In the process of preparing pyriminobac-methyl, high-risk reagents such as n-butyllithiumare avoided, a large amount of wastewater generated by diazotization is avoided, the income is increased, and the environment is protected.
Owner:CHANGZHOU UNIV
Chemical derivation-based detection method for hemolymph metabolin of migratory locust
The invention discloses a chemical derivation-based detection method for hemolymph metabolin of migratory locust, and relates to the field of animal ecology and analytical chemistry. The detection method specifically comprises the following steps of: taking hemolymph, deproteinizing by adding ethanol-acetonitrile mixed solvent, supersonically extracting, and centrifuging at a high speed to obtain metabolin extracting solution; drying the extracting solution by means of pressure reduction, adding methoxamine hydrochloride-pyridine solution, supersonically dissolving, blending by means of vortex, and deriving in an oximation way in a constant-temperature water bath; adding N-methyl-N-trimethylsilane-trifluoroacetamide reagent, blending by means of vortex, and deriving in an alkylation way in the constant-temperature water bath; and adding methyl oleate-normal heptane solution to compensate the volume, blending by means of vortex, and analyzing by a spare gas chromatography-mass spectra coupling technique. The detection method provided by the invention is convenient, high-efficiency, mild in reaction conditions, good in repeatability, wide in metabolin coverage, suitable for developing the multi-center and large-sample research, and capable of providing help for knowing the biological law of various migratory locust variations and the occurrence mechanism of the locust plague.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of methoxyamine hydrochloride and preparation method of N-methoxyacetamide
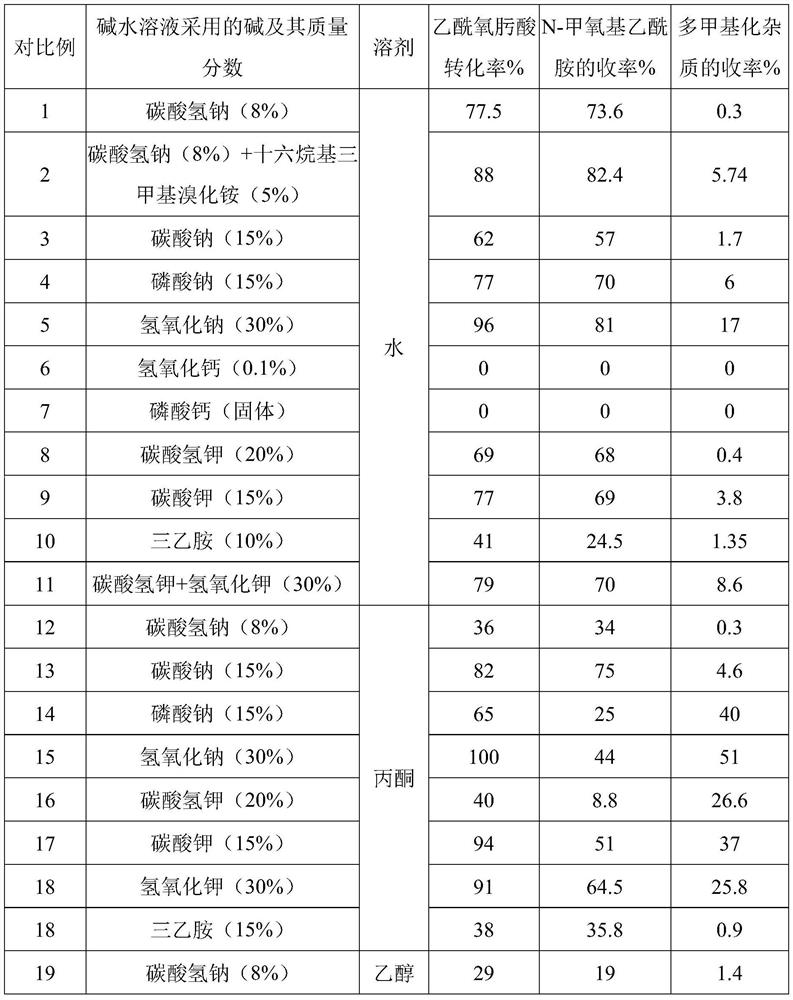
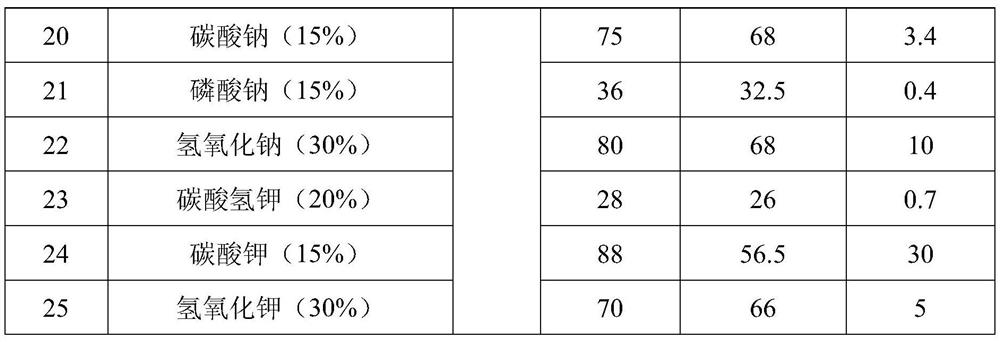
The invention relates to a preparation method of methoxyamine hydrochloride and a preparation method of N-methoxyacetamide, belonging to the technical field of organic synthesis. The preparation method of the methoxyamine hydrochloride comprises the following steps: 1) carrying out a methylation reaction on acetohydroxamic acid and dimethyl sulfate in water to generate N-methoxyacetamide, wherein in the process of the methylation reaction, sodium bicarbonate and sodium hydrogen salt are adopted to control the pH value of a reaction system to be 7-9, and a molar ratio of sodium bicarbonate to sodium hydroxide is (0.03-0.09): 1; and 2) preparing methoxyamine hydrochloride from N-methoxyacetamide. According to the preparation method of methoxyamine hydrochloride, composite alkali of sodium bicarbonate and sodium hydroxide is adopted in the methylation reaction process, so the conversion rate of acetohydroxamic acid can be increased, the pH value in the methylation process can be strictly controlled to be 7-9, generation of polymethylation impurities (O,N-dimethylhydroxylamine hydrochloride) is reduced, and the yield of N-methoxyacetamide is improved.
Owner:ZHENGZHOU SIGMA CHEM
A kind of synthetic method of Relugoli
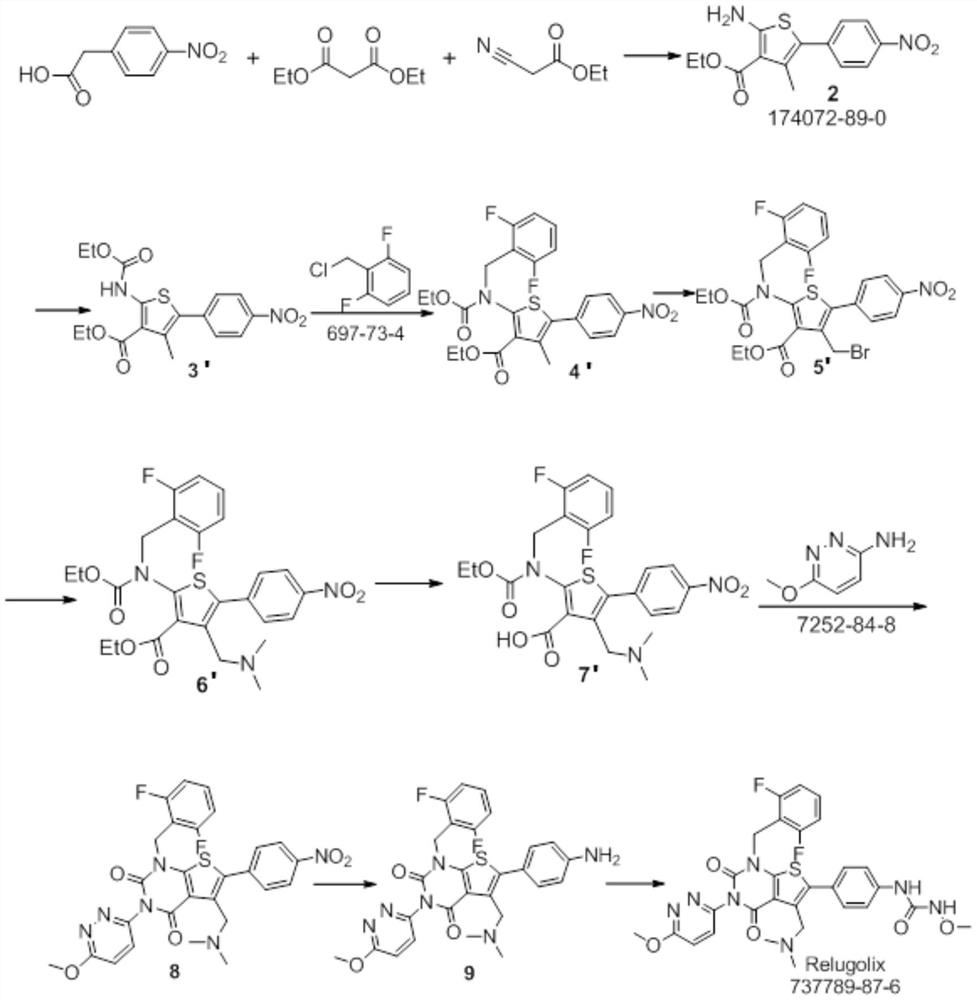
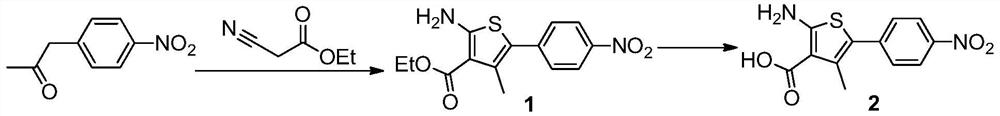
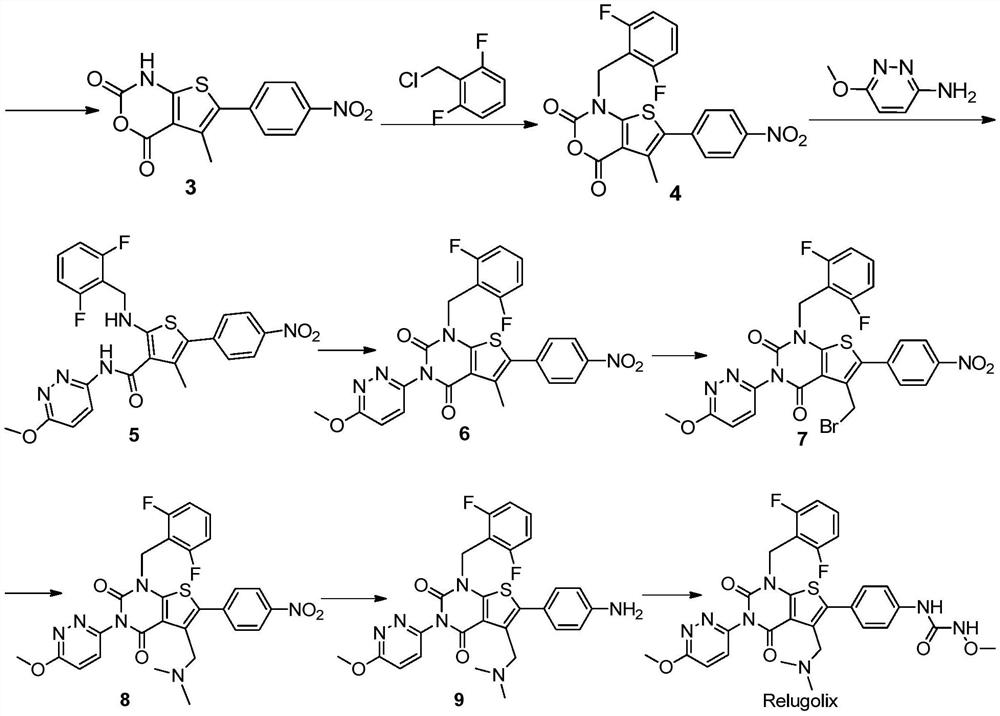
The present invention provides a method for preparing relugoli intermediate compound 8, said method comprising the following steps: (a) reacting compound 2 with N,N'-carbonyldiimidazole to obtain compound 3; (b) compound 3 React with 2,6-difluorobenzyl chloride to give compound 4; (c) react compound 4 with 3-amino-6-methoxypyridazine to give compound 5; (d) react compound 5 with N,N'-carbonyl Diimidazole reacts to obtain compound 6; (e) compound 6 reacts with N-bromosuccinimide and azobisisobutyronitrile to obtain compound 7; (f) compound 7 reacts with dimethylamine hydrochloride, Compound 8 was obtained. The present invention also provides a method for preparing relugoli, said method comprising the following steps: (g) reacting compound 8 prepared by the above method with hydrogen under a catalyst to obtain compound 9; (h) compound 9 and N , N'‑carbonyldiimidazole and methoxylamine hydrochloride were reacted to obtain relugoli. The method for preparing relugoli provided by the present invention adopts the route of first ring closure and then coupling, which has simpler operation, fewer side reactions, mild reaction conditions, high yield and purity, easy purification of the product, and is suitable for commercial scale production.
Owner:四川伊诺达博医药科技有限公司
A kind of refining method of methoxyamine hydrochloride
ActiveCN113185426BHigh purityDoes not affect continuityMembranesOrganic chemistryInorganic saltsChemical industry
The present invention relates to the field of chemical industry. The present invention is a method for refining methoxyamine hydrochloride. First, the volatile organic matter and reaction raw materials in the reaction liquid are rectified and removed by rectification, and then concentrated by a concentration tower and then added The organic solvent causes the inorganic salt to precipitate, and the inorganic salt in the kettle liquid is removed by filtration; what this method uses is a kind of hydrophilic polysulfone microporous filtration membrane, which has good filtration effect and filtration efficiency, and does not affect the process of the whole process. Continuity; the present invention uses the principle of the different solubility of methoxyamine hydrochloride and inorganic salts in organic solvents to precipitate inorganic salts by adding organic solvents, and then achieve the purpose of removing inorganic salts; organic solvents of the present invention can be recycled , do not increase the material cost of production, do not increase the treatment capacity of the three wastes, and the methoxyamine hydrochloride purified by the method of the present invention has higher purity, which improves product quality and market competitiveness.
Owner:ZHEJIANG JINHUA NEW MATERIALS
A kind of method adopting microreactor to prepare methoxyamine hydrochloride
ActiveCN113045451BAccurate control precisionReact SafeOrganic chemistryChemical/physical/physico-chemical microreactorsHydroxylamineDistillation
Owner:南京科力硕生物科技有限公司
Benzenesulfonylation reagent as well as preparation method and application thereof
ActiveCN106905196ASave raw materialsEasy to makeSulfonic acid esters preparationSulfonic acid amide preparationSide effectTwo step
The invention discloses a benzenesulfonylation reagent. The structure of the benzenesulfonylation reagent is shown as formula I in the specification, wherein R is a benzene ring connected with 0-5 substituents, and the substituents on the benzene ring are chlorine atoms, fluorine atoms, methyl groups or tert-butyl groups independently. The invention further discloses a preparation method and an application of the benzenesulfonylation reagent. The benzenesulfonylation reagent is prepared from methoxyamine hydrochloride and benzenesulfonyl chloride or a benzenesulfonyl chloride derivative through two steps of reactions, raw materials are cheap, a preparation process is simple, reaction conditions are mild, few side effects are produced, only extraction and simple column chromatographic purification are needed for aftertreatment, and the yield is high. The benzenesulfonylation reagent has higher benzenesulfonylation activity, benzenesulfonylation reaction conditions are mild, a reaction substrate range is wider, compounds such as alcohol, phenol, naphthol, thiophenol and the like can be subjected to a benzenesulfonylation reaction in presence of acetonitrile neutralized potassium carbonate, and -C1 does not exist on the benzenesulfonylation reagent, so that HCl gas with pungent odor cannot be emitted during application.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Preparation method of decarbamyl cefuroxime
The invention provides a preparation method of decarbamoyl cefuroxime. According to the method, 7-ACA or D-7-ACA is taken as a starting material and is subjected to one-step reaction with carbon monoxide and a compound I under the catalysis of metal palladium to obtain a decarbamoyl cefuroxime intermediate ketoamide, and the intermediate reacts with methoxyamine hydrochloride to obtain a product decarbamoyl cefuroxime, wherein the HPLC purity is 98.0% or above. The method has the characteristics of short route, high yield, high purity, less three wastes and suitability for industrial production.
Owner:GUANGDONG LIGUO PHARMACY
A kind of synthetic method of 4-allyl-3,5-disubstituted isoxazole
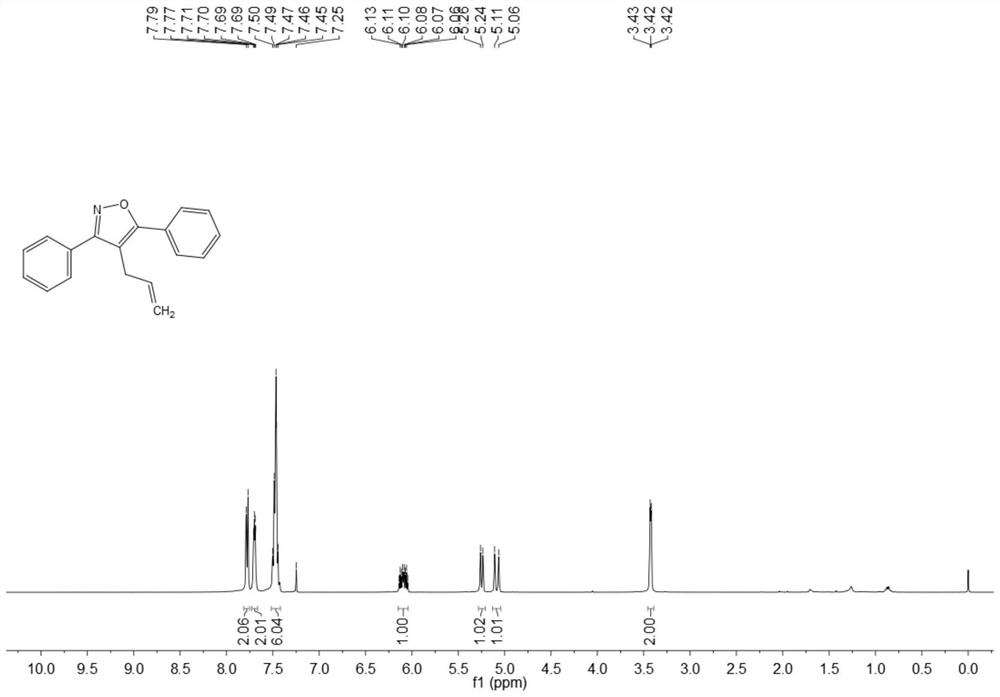
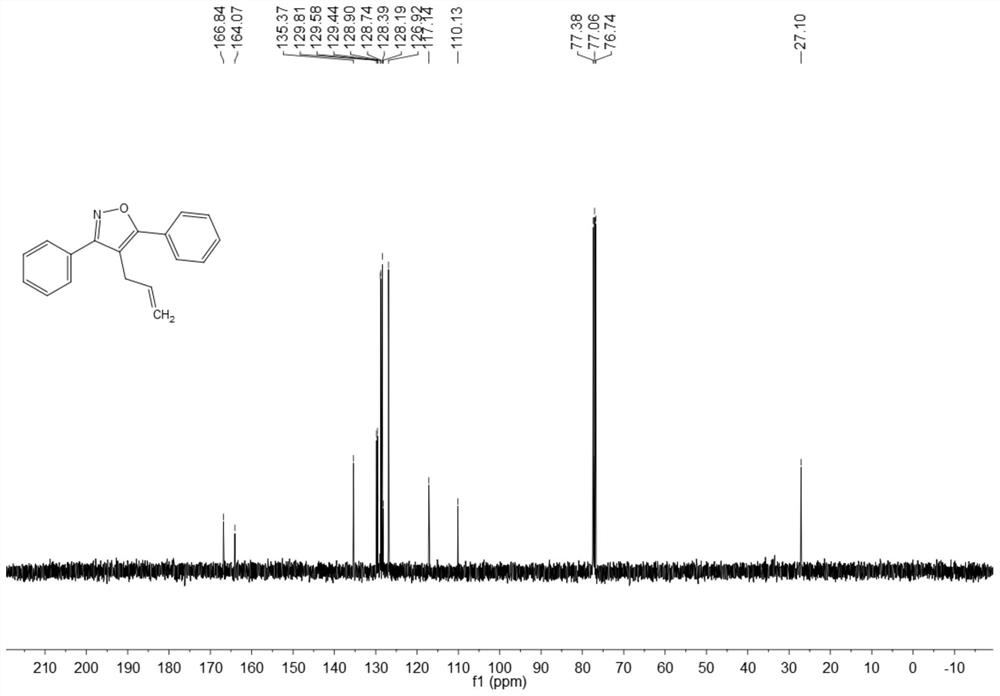
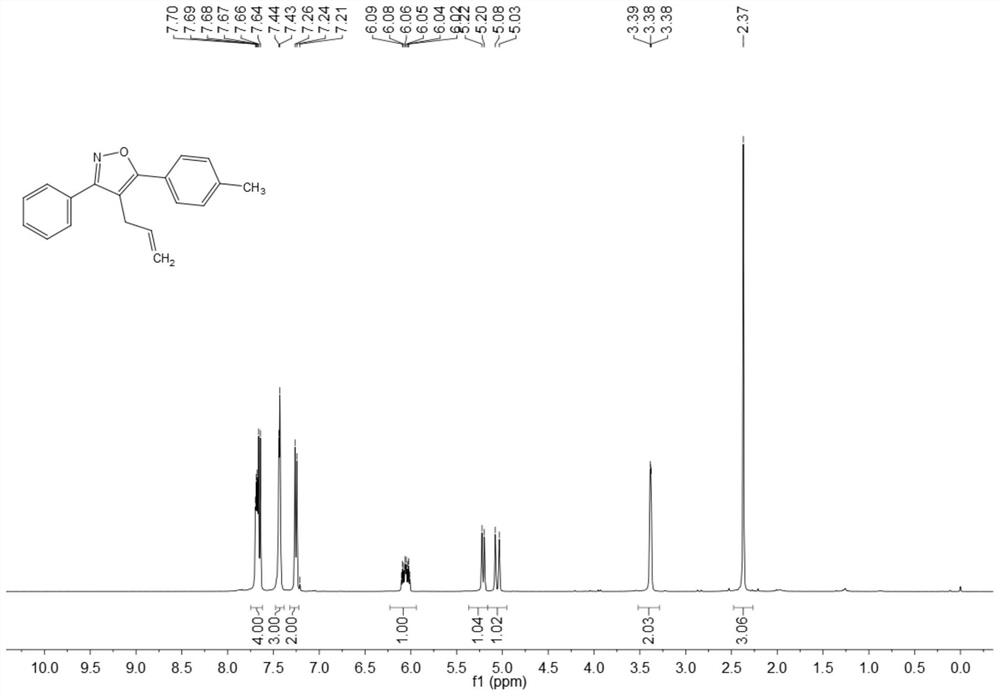
ActiveCN108863969BRaw materials are easy to getInnovativeOrganic chemistryPtru catalystOrganic synthesis
The invention discloses a synthesis method of 4-allyl-3,5-disubstituted isoxazole, belonging to the technical field of organic synthesis. The synthesis method is as follows: in the reactor, add acetylene ketoxime ether substrate, 3-bromopropene, palladium catalyst, additive and solvent, stir and react at 70-80°C, and the reaction product is separated and purified to obtain 4-allyl -3,5-disubstituted isoxazoles. In the method of the present invention, a series of acetylenic ketone oxime ethers are obtained by reacting the product obtained through Sonogashira coupling of simple and easy-to-obtain acyl chlorides and alkynes with methoxyamine hydrochloride, the reaction conditions are mild, and there is no pollution to the environment. Potentially functional 4-allyl-3,5-disubstituted isoxazole compounds, the method is innovative and atom-economical, mild conditions, safe operation, and can be scaled up to 5 g scale without affecting the yield Therefore, it has potential practical value.
Owner:SOUTH CHINA UNIV OF TECH
The preparation method of zabufloxacin intermediate
InactiveCN108623608BHigh yieldQuality improvementOrganic chemistryAcrylonitrileMethanesulfonyl chloride
The invention provides a preparation method of a zabofloxacin intermediate. The method comprises: reacting ethyl glycinate hydrochloride with acrylonitrile under catalysis of a base to form an intermediate I; then reacting the intermediate I with Boc anhydride under the function of a strong base to generate an intermediate III; reacting the intermediate III, the methoxyamine hydrochloride and formaldehyde to generate an intermediate IV; and then preparing the zabofloxacin intermediate by reacting the intermediate IV with methanesulfonyl chloride, performing reduction with sodium borohydride, and other steps. The zabofloxacin intermediate is prepared through nine steps with a high yield and high quality by the method, the material adding amount reaches the level of hundreds of grams, and the method is suitable for industrial production.
Owner:沈阳林特制药有限公司
Preparation method of gemifloxacin side chain compound
ActiveCN113773240ASimple processMild reaction conditionsOrganic chemistryBulk chemical productionPalladium on carbonTert-Butyloxycarbonyl protecting group
The invention discloses a preparation method of a gemifloxacin side chain compound. The preparation method comprises the following steps: S1, carrying out a reaction on 1-N-tert-butyloxycarbonyl-4-cyano-3-pyrrolidone and methoxyamine hydrochloride to obtain a compound as shown in a formula (V); S2, in an inert atmosphere, at a temperature of 10-50 DEG C, in the presence of palladium on carbon, and in a hydrogen atmosphere of 0.1-0.5 Mpa, carrying out a hydrogenation reduction reaction on the compound as shown in the formula (V) for 1-3 h; adding Boc anhydride, continuing the hydrogenation reduction reaction for 4-8 h under the conditions of 10-50 DEG C and 0.1-0.5 Mpa, and obtaining a compound shown in the formula VI; S3, enabling the compound shown in the formula VI to react with acid to remove the protecting group, and obtaining the gemifloxacin side chain compound shown in the formula I. The preparation method disclosed by the invention is simple in process, very mild in reaction condition and very easy to implement; (2) the raw materials are low in price and cost; (3) a novel and effective gemifloxacin side chain synthesis process route is created.
Owner:北京阳光诺和药物研究股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com