Preparation method of 4-bromo-5-methyl-1H-indazole
A technology of methyl and indazole, which is applied in the field of preparation of 4-bromo-5-methyl-1H-indazole, can solve the problems of easy flushing, high production cost, and danger, and achieve low pollution and easy handling Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
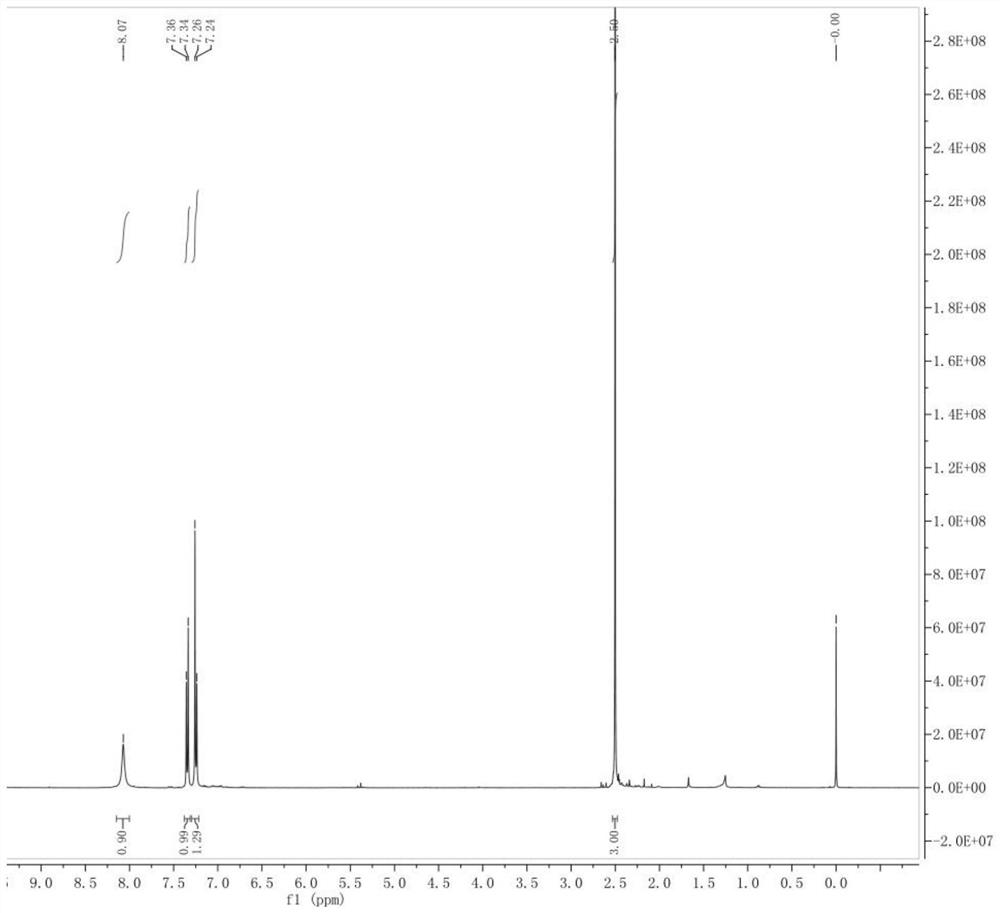
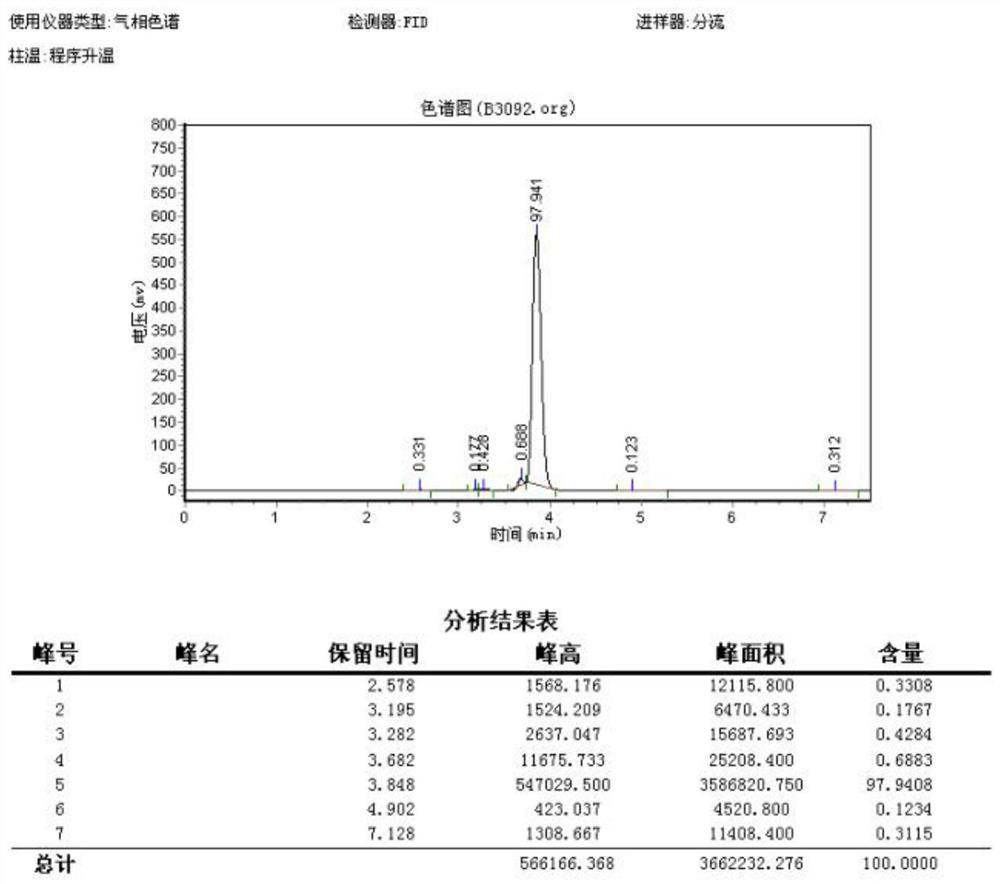
Image
Examples
Embodiment 1
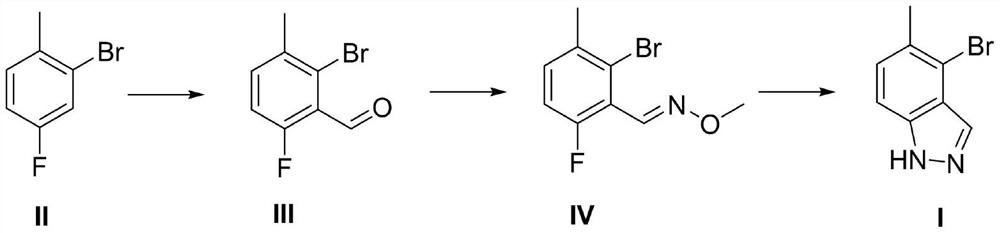
[0024] A kind of preparation method of 4-bromo-5-methyl-1H-indazole, described preparation method comprises the steps:
[0025] (1) Synthesis of compound III
[0026] Add 189g (1mol) 2-bromo-4-fluorotoluene and 1000ml tetrahydrofuran to the reaction flask, cool down to -78°C, add 500ml (1mol) 2.0M LDA solution dropwise, keep the temperature for 2 hours after the dropwise addition, drop Add 73g (1mol) of N,N-dimethylformamide, slowly return to room temperature and stir for 2 hours after dropping, and follow the reaction by HPLC until the reaction of 2-bromo-4-fluorotoluene is complete; quench into saturated ammonium chloride aqueous solution, 2000ml of ethyl acetate was extracted and separated, and the organic phase was dried and concentrated to obtain 190g of compound III with a molar yield of 87.6%.
[0027] (2) Synthesis of Compound IV
[0028] Add 108.5g (500mmol) of compound III, 41.8g (500mmol) of methoxyamine hydrochloride, 69g (500mmol) of potassium carbonate, and 500...
Embodiment 2
[0034] A kind of preparation method of 4-bromo-5-methyl-1H-indazole, described preparation method comprises the steps:
[0035] (1) Synthesis of compound III
[0036] Add 189g (1mol) 2-bromo-4-fluorotoluene and 1000ml tetrahydrofuran to the reaction flask, cool down to -78°C, add 600ml (1.2mol) 2.0M LDA solution dropwise, keep the temperature for 1.5 hours after the dropwise addition, Add 87.6g (1.2mol) N, N-dimethylformamide dropwise, slowly return to room temperature and stir for 1.5 hours after dropping, HPLC follows the reaction until the reaction of 2-bromo-4-fluorotoluene is complete; quenched to saturated ammonium chloride In the aqueous solution, 2000ml of ethyl acetate was extracted and separated, and the organic phase was dried and concentrated to obtain 195g of compound III, with a molar yield of 89.9%.
[0037] (2) Synthesis of Compound IV
[0038] Add 108.5g (500mmol) compound III, 50.1g (600mmol) methoxylamine hydrochloride, 82.8g (600mmol) potassium carbonate,...
Embodiment 3
[0042] A kind of preparation method of 4-bromo-5-methyl-1H-indazole, described preparation method comprises the steps:
[0043] (1) Synthesis of compound III
[0044] Add 189g (1mol) 2-bromo-4-fluorotoluene and 1000ml tetrahydrofuran to the reaction flask, cool down to -78°C, add 750ml (1.5mol) 2.0M LDA solution dropwise, keep the temperature for 1 hour after the dropwise addition, Add 109.5g (1.5mol) N, N-dimethylformamide dropwise, slowly return to room temperature and stir for 1 hour after dropping, HPLC follows the reaction until the reaction of 2-bromo-4-fluorotoluene is complete; quenched to saturated ammonium chloride In the aqueous solution, 2000ml of ethyl acetate was extracted and separated, and the organic phase was dried and concentrated to obtain 198g of compound III, with a molar yield of 91.3%.
[0045] (2) Synthesis of Compound IV
[0046] Add 108.5g (500mmol) compound III, 62.7g (750mmol) methoxyamine hydrochloride, 103.5g (750mmol) potassium carbonate, 500m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com