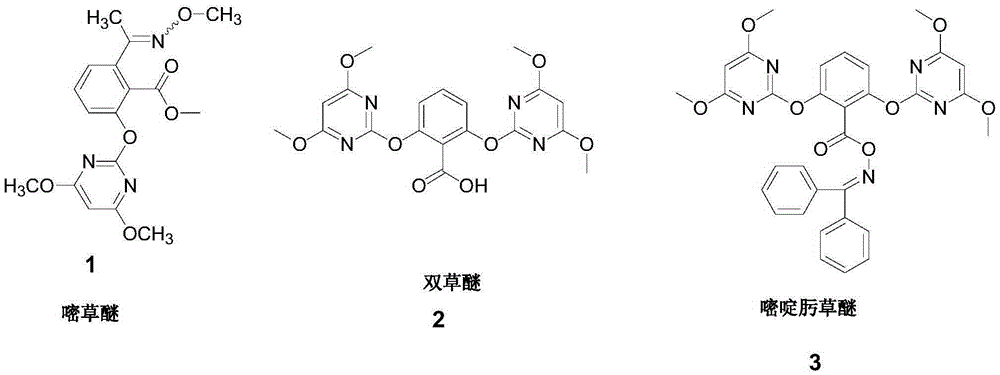
Preparation method of paddy field herbicide pyriminobac-methyl
A technology of pyriflubacin and organic solvents, applied in the field of agrochemicals, can solve problems such as the complex process of pyriflubacin, and achieve the effects of mild reaction, high yield and good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
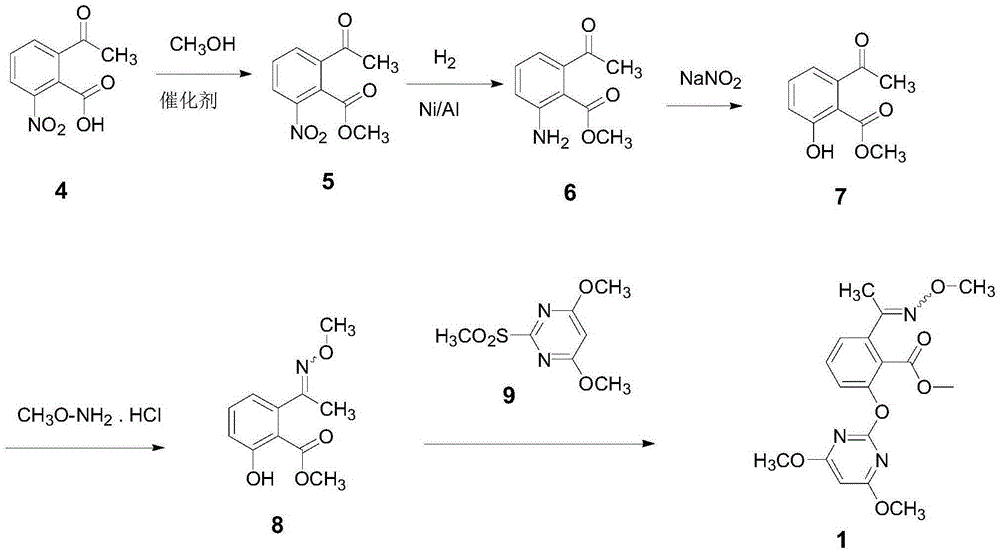
[0030] Example 1: Preparation of 2-acetyl-6-nitrobenzoic acid methyl ester
[0031] Add 2-acetyl-6-nitrobenzoic acid (52.4g, 0.25mol), methanol (500mL) and p-toluenesulfonic acid (1.2g, 10.0mmol) into a 1L reaction flask, and heat to reflux for 8h under stirring , after the reaction, methanol was distilled off under reduced pressure, and the residue was recrystallized twice from toluene, and dried to obtain light yellow solid methyl 2-acetyl-6-nitrobenzoate (50.1 g, yield 89.8%), melting point 75.2 -77.4°C,
[0032] 1 HNMR (300MHz, CDCl 3 )δ8.32(dd, J=8.3,1.0Hz,1H),8.12(dd,J=7.8,1.0Hz,1H),7.72(t,J=8.0Hz,1H),4.01(s,3H), 2.66(s,3H).
example 2
[0033] Example 2: Preparation of 2-acetyl-6-aminobenzoic acid methyl ester
[0034] Add 2-acetyl-6-nitrobenzyl ester (50.0g, 0.22mol), nickel-aluminum catalyst (5.0g) and ethyl acetate (500mL) prepared in Example 1 in a 1L autoclave, stirring Under the pressure of hydrogen gas, the pressure was 50 atm at room temperature (25 ℃) to react for 8 hours. After the reaction, the catalyst was recovered by filtration, the filtrate was concentrated to recover ethyl acetate, and the residue was recrystallized with methanol to obtain light yellow crystals, 2-acetyl-6- Methyl aminobenzoate (41.1g, yield 94.9%), melting point 137.5-139.2°C,
[0035] 1 HNMR (300MHz, CDCl 3 )δ7.25(dd, J=7.9,7.2Hz,1H),6.76(d,J=8.3Hz,1H),6.62(d,J=7.3Hz,1H),5.11(s,2H),3.83( s,3H), 2.45(s,2H).
example 3
[0036] Example 3: Preparation of 2-acetyl-6-hydroxybenzoic acid methyl ester
[0037] Add 2-acetyl-6-aminobenzoic acid ester (40.0g, 0.20mol) prepared in Example 2 and a sulfuric acid solution (300mL) with a solute mass fraction of 20% in a 1L reaction flask, cool to 0°C, Add 200 mL of sodium nitrite aqueous solution (containing 15.0 g of sodium nitrite, 0.22 mol) dropwise under stirring to prepare a diazonium salt solution for later use, and the time for dropping is 2 hours.
[0038] Add cyclohexane (400ml) and sulfuric acid solution (400mL) with a mass fraction of solute of 25% in a 2L reaction flask, heat to 85°C and add the above-mentioned diazonium salt solution dropwise therein for 1 hour. After the completion of the reaction, the reaction was continued for 4 hours. After the reaction was completed, it was cooled, extracted with cyclohexane (200ml*3), concentrated, and the residue was recrystallized with cyclohexane to obtain a light yellow solid methyl 2-acetyl-6-hydrox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com