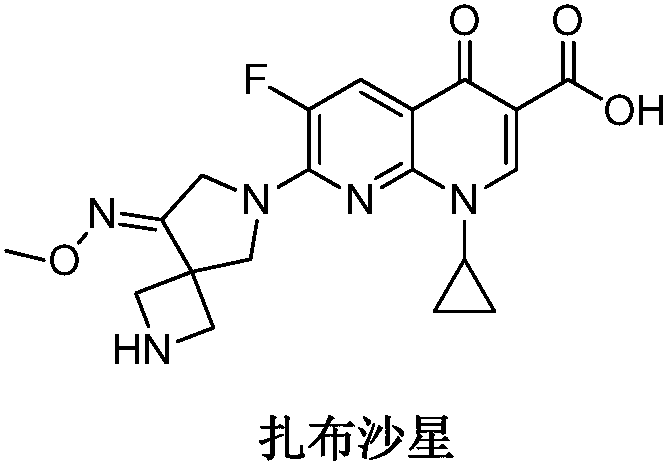
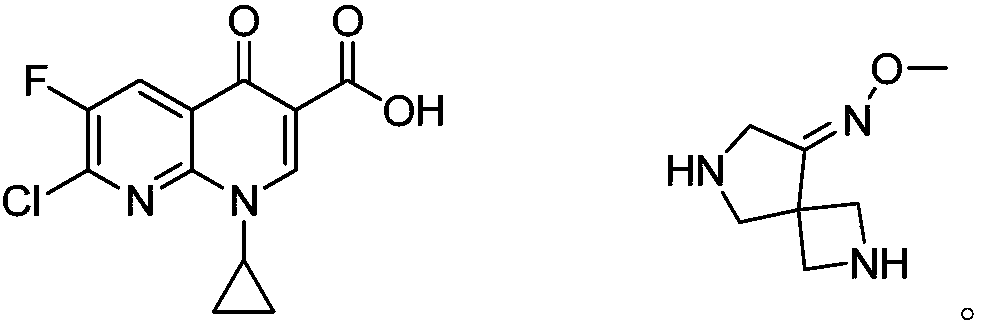
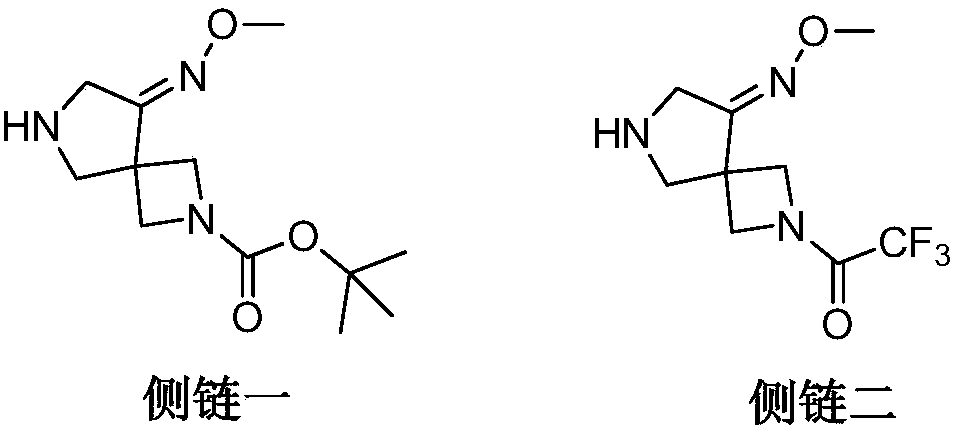
Preparation method of zabofloxacin intermediate
A technology for zabfloxacin and intermediates, which is applied in the field of preparation of zabfloxacin intermediates, can solve the problems of unsuitability for industrial production, low yield and high synthesis cost, and achieves simplified operation, improved product quality and huge profit margins. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Preparation of tert-butyl 3-cyano-4-oxo-pyrrolidine-1-carboxylate (intermediate III)
[0046] The reaction equation is as follows:
[0047]
[0048] Feeding ratio:
[0049]
[0050] crafting process:
[0051] At room temperature, add 500g of glycine ethyl ester hydrochloride, 172.8g of NaOH and 1000g of methanol into the reaction flask and stir for 1 hour, cool the reaction solution to below 10°C, and add 257.4g of acrylonitrile dropwise to the reaction system under temperature control below 10°C 500g of methanol solution, drop it in about 2h, raise the temperature of the reaction solution to room temperature and continue to stir for 1h, then raise the temperature of the reaction solution to 65°C, reflux and stir for 5h, and monitor the completion of the reaction by thin-layer chromatography (ninhydrin baking plate method ), the reaction solution was cooled to below 40°C, the temperature was controlled below 40°C and the methanol was concentrated under...
Embodiment 1-1
[0054] Example 1-1 Preparation of tert-butyl 3-cyano-4-oxo-pyrrolidine-1-carboxylate (intermediate III) Feed ratio:
[0055]
[0056]
[0057] crafting process:
[0058] At room temperature, add 500g of glycine ethyl ester hydrochloride, 158.4g of NaOH and 1000g of methanol into the reaction flask and stir for 1h, cool the reaction solution to 5°C, and add 248g of acrylonitrile and 500g of Methanol solution, after about 2 hours of dripping, the temperature of the reaction solution was raised to room temperature and continued stirring for 1 hour, and then the temperature of the reaction solution was raised to 70°C, and stirred at reflux for 5 hours, and the reaction was monitored by thin-layer chromatography (ninhydrin baking plate method). The temperature of the reaction solution was lowered to below 40°C, the temperature was controlled below 40°C, and the methanol was concentrated under reduced pressure to obtain 570 g of crude intermediate I as a light yellow oil. The ...
Embodiment 1-2
[0061] Example 1-2 Preparation of tert-butyl 3-cyano-4-oxo-pyrrolidine-1-carboxylate (intermediate III)
[0062] Feeding ratio:
[0063]
[0064]
[0065] crafting process:
[0066] At room temperature, add 500g of glycine ethyl ester hydrochloride, 187.2g of NaOH and 1200g of methanol into the reaction flask and stir for 1h. After about 2 hours of dripping, the reaction solution was raised to room temperature and continued to stir for 0.5 hours, then the reaction solution was raised to 68°C, refluxed and stirred for 5 hours, and the reaction was monitored by thin-layer chromatography (ninhydrin baking plate method) , the temperature of the reaction solution was lowered to below 40°C, the temperature was controlled below 40°C, and the methanol was concentrated under reduced pressure until it was purified to obtain 575g of crude intermediate I as a light yellow oil.
[0067] Cool the oil obtained in the previous step to 2°C, add 1020.2g of Boc anhydride, after the addit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com