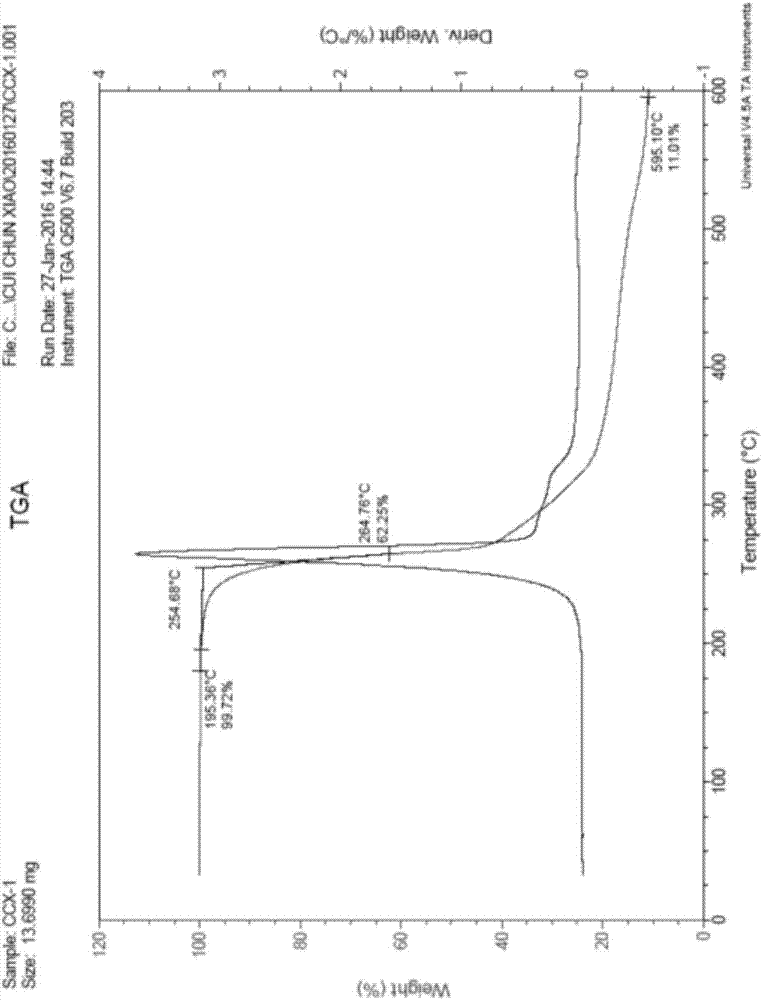
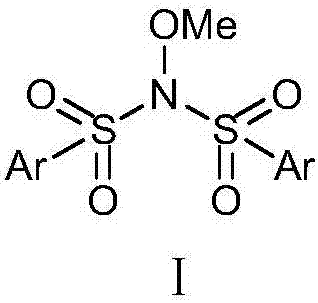
Benzenesulfonylation reagent as well as preparation method and application thereof
A technology for benzenesulfonyl and reagents, applied in the field of benzenesulfonyl reagents and its preparation, achieves the effects of high yield, simple preparation process and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 benzenesulfonylation reagent II
[0032] Synthetic route of benzenesulfonylation reagent Ⅱ
[0033]
[0034] Synthesis of compound b1
[0035] Add water (75mL), ethyl acetate (75mL), benzenesulfonyl chloride (20.0g, 113mmol), potassium carbonate (34.48g, 2.2eq.), methoxyamine hydrochloride (11.4g, 1.2 eq.), reacted at room temperature (20-30°C) overnight (about 12 hours), extracted with ethyl acetate, and spin-dried the solvent to obtain a white solid, namely compound b1 (20.18 g, yield 95%).
[0036] Synthesis of Benzenesulfonylation Reagent Ⅱ
[0037] Add acetonitrile (100mL), compound b1 (20.18g, 108mmol), potassium carbonate (17.7g, 1.2eq.) and benzenesulfonyl chloride (22.6g, 1.2eq.) to a 250mL three-necked flask, and react overnight at room temperature (about 12 hours) , separated by column chromatography after the reaction is complete. The separation type is adsorption column chromatography, the column temperature is normal ...
Embodiment 2
[0039] The benzenesulfonylation of embodiment 2 ethanol
[0040]
[0041] Add acetonitrile (3mL), substrate ethanol (0.014g, 0.3mmol), potassium carbonate (0.041g, 0.3mmol), benzenesulfonylation reagent II (0.082g, 0.25mmol) into a 10mL reaction tube, stir at 80°C, TLC detection reaction. After the reaction was detected by TLC, it was separated by column chromatography. The separation type is adsorption column chromatography, the column temperature is normal temperature (20-30°C), the adsorbent is silica gel, the eluent is petroleum ether and ethyl acetate, gradient elution, the column height-to-diameter ratio is 10:1, dry method Pack the column and add the sample by dry method. The elution rate is 1-2 drops per second. The eluent was PE:EA=10:1 (volume ratio), and the eluate obtained from the separation was rotary evaporated to remove the solvent to obtain the benzenesulfonylated product of ethanol (0.053 g, yield 98%). 1 H NMR (400MHz, CDCl 3 )δ7.86(d, J=7.2Hz, 2H), ...
Embodiment 3
[0042] The benzenesulfonylation of embodiment 3 phenol
[0043]
[0044]Add acetonitrile (3mL), substrate phenol (0.024g, 0.25mmol), potassium carbonate (0.041g, 0.3mmol), benzenesulfonylation reagent II (0.098g, 0.3mmol) into a 10mL reaction tube, stir at 80°C, TLC detection reaction. After the reaction was detected by TLC, it was separated by column chromatography. The separation type is adsorption column chromatography, the column temperature is normal temperature (20-30°C), the adsorbent is silica gel, the eluent is petroleum ether and ethyl acetate, gradient elution, the column height-to-diameter ratio is 10:1, dry method Pack the column and add the sample by dry method. The elution rate is 1-2 drops per second. The eluent was PE:EA=10:1 (volume ratio), and the separated eluate was evaporated to remove the solvent to obtain the benzenesulfonylated product of phenol (0.058 g, yield 99%). 1 H NMR (400MHz, CDCl3) δ7.82(d, J=7.2Hz, 2H), 7.65(t, J=7.6Hz, 1H), 7.51(t, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com