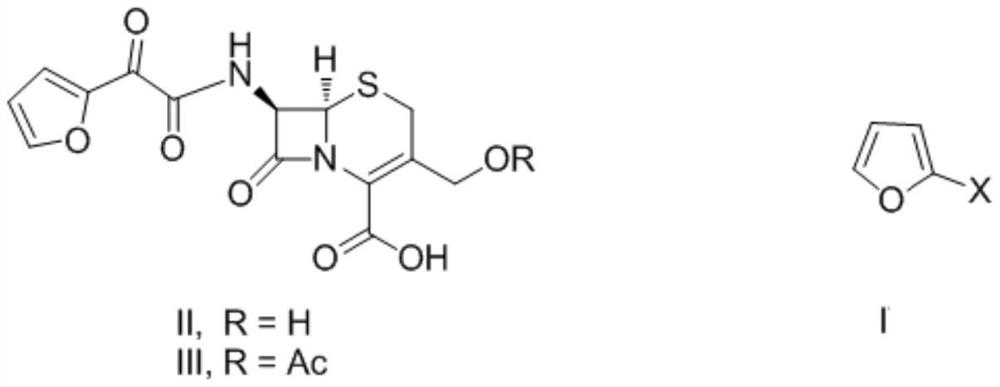
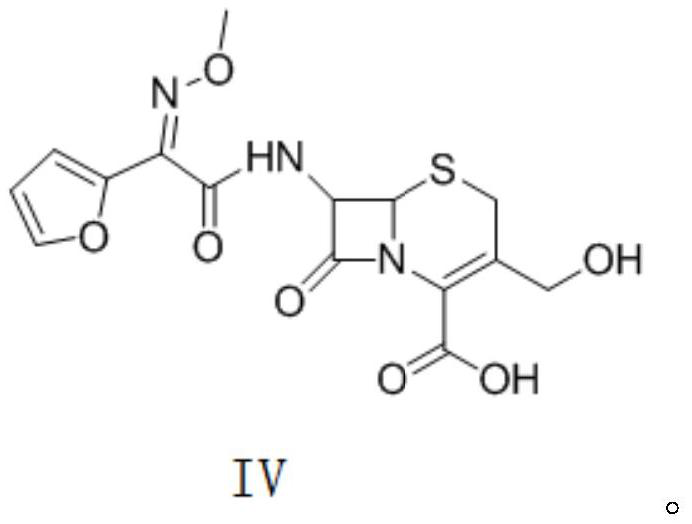
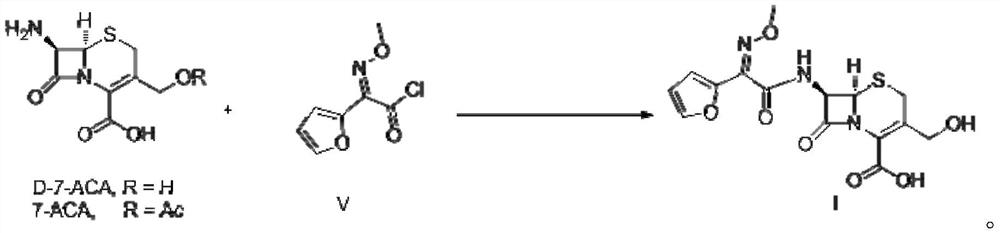
Preparation method of decarbamyl cefuroxime
A technology of carbamoyl cephalosporins and nitroxides, applied in the field of medicines, can solve problems such as hidden dangers of safety and restricting the development of enterprises
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add water (40ml), palladium chloride (0.02mmol), sodium acetate (30mmol), NaI (0.5mmol), 2-bromofuran (10mmol), D-7-ACA (10mmol), nitrogen into 100ml autoclave successively After the replacement, stir the reaction under 1 atmosphere of carbon monoxide at 5-10°C. When HPLC shows that the D-7-ACA residue is less than 0.5%, exhaust the gas and dilute it with nitrogen, and then add dichloromethane to quench the reaction. Stir at 10°C for 30 minutes, stand still for 10 minutes to separate the liquid, collect the water phase, add dichloromethane, stir at 5-10°C for 30 minutes, stand still for 10 minutes, separate the liquid, collect the water phase, and combine the two water phases.
[0030] Under the condition of 5-10°C, add sodium bicarbonate (15mmol) and methoxyamine hydrochloride (11mmol) to the above water phase, and stir the reaction at 5-10°C, when HPLC shows that the residual product of the previous step is less than 0.5%. , add hydrochloric acid to adjust the pH of t...
Embodiment 2
[0032] Add polyethylene glycol, water mixture (40ml, volume ratio 1:2), palladium chloride (0.02mmol), sodium carbonate (20mmol), NaI (0.5mmol), 2-bromofuran ( 10mmol), D-7-ACA (10mmol), after nitrogen replacement, stir the reaction under 2 atmospheres of carbon monoxide at 5-10°C, when HPLC shows that the residual D-7-ACA is less than 0.5%, exhaust the gas and dilute with nitrogen After discharge, add dichloromethane to quench the reaction, stir at 5-10°C for 30 minutes, stand still for 10 minutes to separate the liquid, collect the water phase, add dichloromethane, stir at 5-10°C for 30 minutes, stand still for 10 minutes, separate the liquid, and collect water box.
[0033] Under the condition of 5-10°C, add sodium bicarbonate (15mmol) and methoxyamine hydrochloride (10mmol) to the above water phase, and stir the reaction at 5-10°C, when HPLC shows that the residual product of the previous step is less than 0.5%. , add hydrochloric acid to adjust the pH of the system to 1-...
Embodiment 3
[0035] Add water (40ml) successively in 100ml autoclave, palladium chloride (0.02mmol), sodium hydroxide (20mmol), NaI (0.5mmol), 2-bromofuran (10mmol), D-7-ACA (10mmol), After nitrogen replacement, stir the reaction under 2 atmospheres of carbon monoxide at 5-10°C. When HPLC shows that the D-7-ACA residue is less than 0.5%, exhaust the gas and dilute it with nitrogen, then add dichloromethane to quench the reaction. Stir at ~10°C for 30 minutes, stand still for 10 minutes to separate the liquid, collect the water phase, add dichloromethane, stir for 30 minutes at 5-10°C, stand still for 10 minutes, separate the liquid, and collect the water phase.
[0036] Under the condition of 5~10 DEG C, add sodium carbonate (15mmol), methoxylamine hydrochloride (10mmol) to above-mentioned water phase, stir reaction under 5~10 DEG C, when HPLC shows that the residual product of the previous step is less than 0.5%, Add hydrochloric acid to adjust the pH of the system to 1-5, and crystals ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com