Method for separating and purifying 9-nitro camptothecin
A technology for separation and purification of nitrocamptothecin, applied in the field of high-purity 9-nitrocamptothecin, which can solve the problems of difficult amplification, cumbersome process, harsh operating environment, etc., and achieve the effect of low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
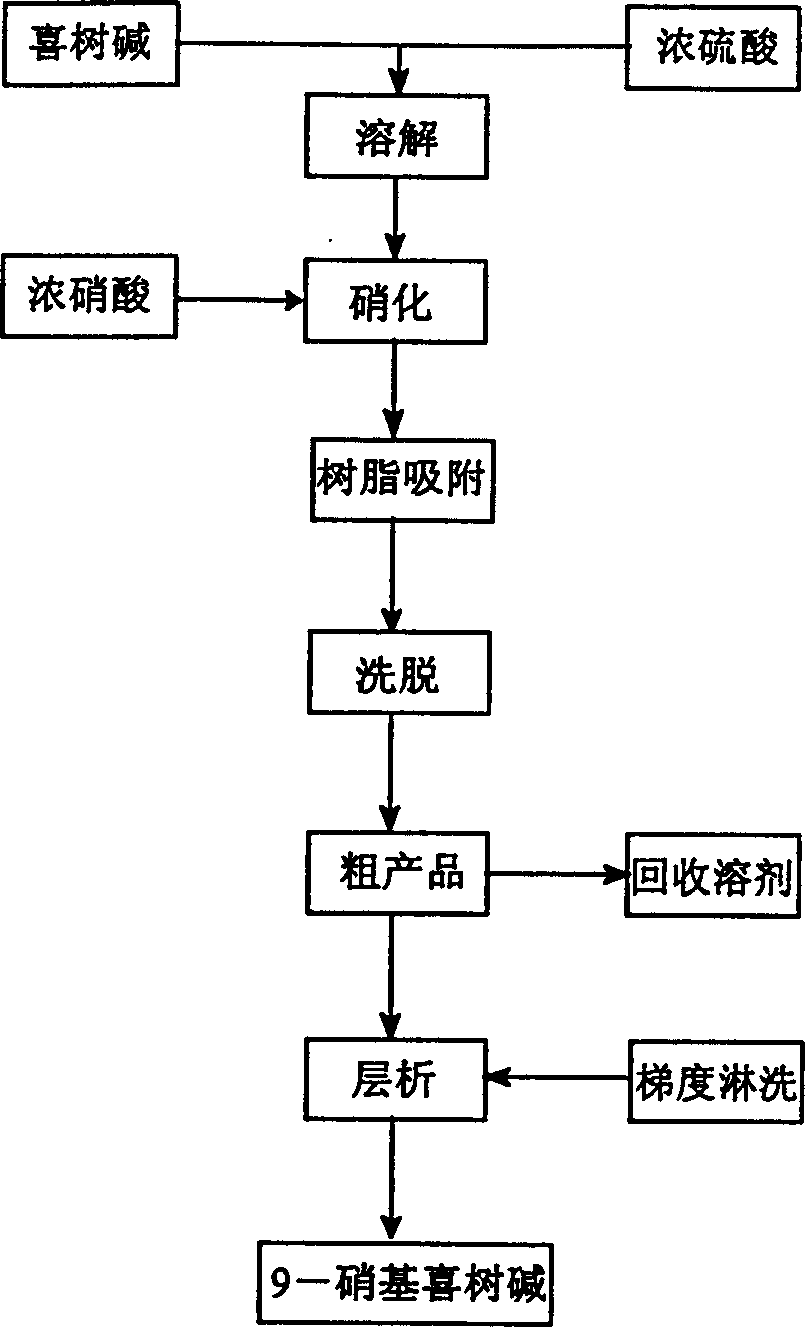
[0022] Add 1500ml of concentrated sulfuric acid to a 2000ml flask, and place it in an ice-salt bath to cool to an internal temperature of -5°C. 5 grams (0.0143 mole) of camptothecin powder were added in batches under stirring, and the reaction liquid was orange-red. After the dissolution is complete, slowly add 4.5ml of concentrated nitric acid (65% to 68%) dropwise under stirring, and the reaction solution turns black after adding half an hour. After the addition was complete, stir at -5°C for one hour, then slowly warm up to room temperature, and stop the reaction after stirring for 72 hours. The reaction solution was poured into 2000 g of ice water to obtain a mixed acid solution of camptothecin nitration product for later use.
[0023] About 300 grams of the processed macroporous adsorption resin HZ-802 (treated according to the conventional treatment method of ion exchange resin and adsorption resin) is packed into a glass column with a diameter of 5 cm and a length of 6...
Embodiment 2
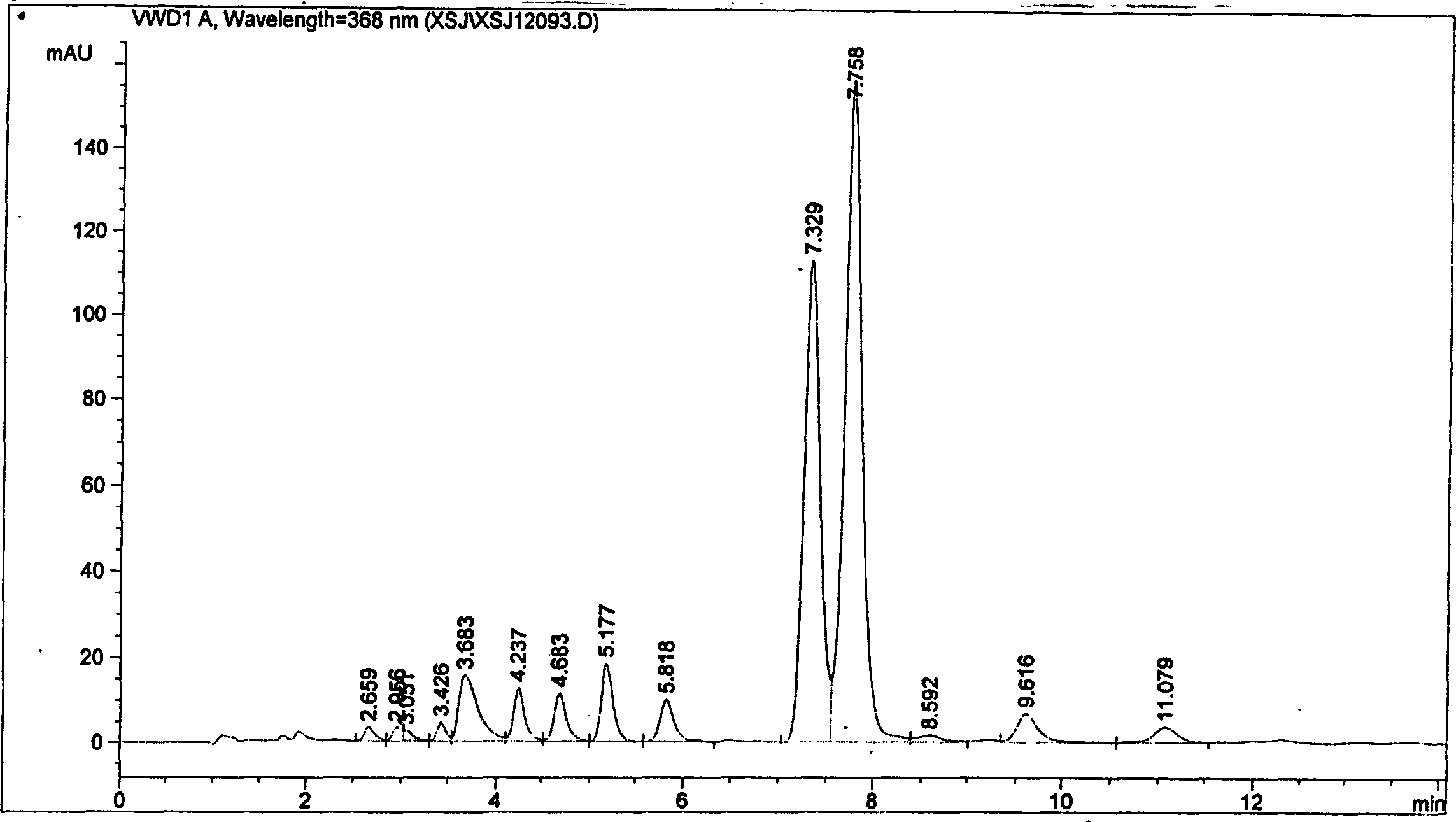
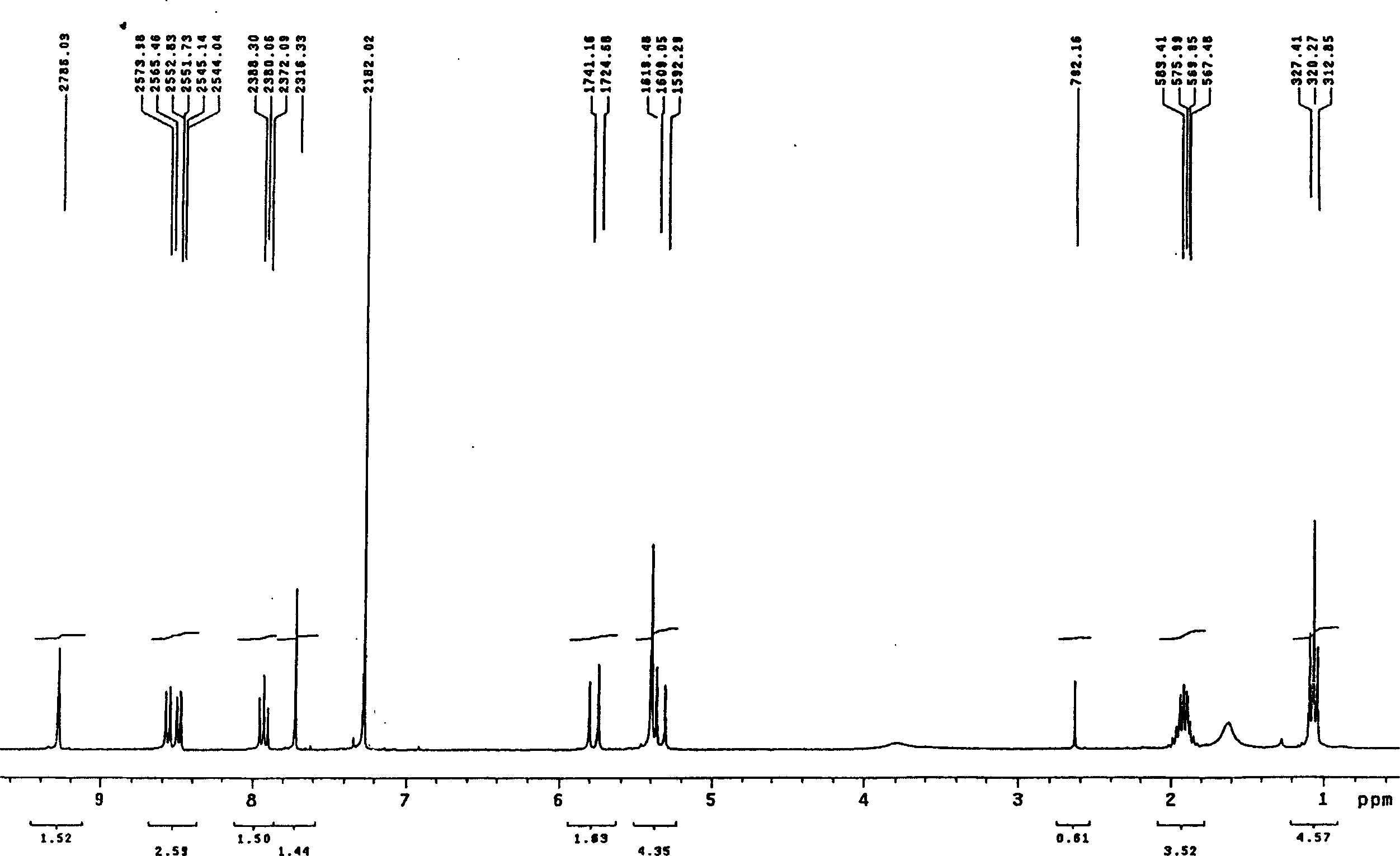
[0026] The mixed acid solution of the camptothecin nitration product was obtained in the same manner as in Example 1, the macroporous adsorption resin was HZ-816, and the eluent was acetone. Silica gel chromatography separation silica gel adopts 200-400 mesh chromatography silica gel, and the eluent ratio is changed to: a) 500ml of n-hexane; b) 1000ml of n-hexane: ethyl acetate is a 4:3 mixture; c) 500ml of n-hexane Alkane: ethyl acetate is a mixed solution of 2: 3; d) 500ml of ethyl acetate rinses, others are operated by the same equipment and process steps as in Example 1, and part b of the rinse solution can obtain a yellow powder through vacuum distillation 1.2 g, its melting point is 275.5-277 ° C, and its purity by high pressure liquid chromatography is 99.7%.
Embodiment 3
[0028] The mixed acid solution of the camptothecin nitration product was obtained in the same manner as in Example 1, the macroporous adsorption resin was HZ-841, and the eluent was methanol. The eluent ratio of the silica gel chromatography separation is changed to: a) 500ml of n-hexane; b) 1000ml of n-hexane: ethyl acetate is a 6:5 mixture; c) 500ml of n-hexane: ethyl acetate is 2:3 d) the ethyl acetate rinse of 500ml, others are operated by the same equipment and process steps as in Example 1, and the b part of the rinse solution can obtain 1.3 grams of yellow powder through vacuum distillation, and its melting point is 275.5-277 °C, and the purity was 99.6% as tested by high pressure liquid chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com