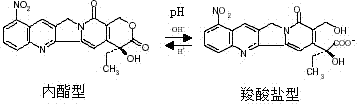
9-nitrocamptothecin-cyclodextrin inclusion compound and preparation method thereof as well as pharmaceutical composition containing cyclodextrin inclusion compound
A technology of cyclodextrin inclusion compound and nitrocamptothecin, which can be used in drug combinations, medical preparations containing active ingredients, antineoplastic drugs, etc., and can solve problems such as low bioavailability and poor solubility of 9-NC , to achieve the effect of improving dissolution rate, solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19-N
[0032] Phase solubility curve experiment of embodiment 19-NC and hydroxypropyl-β-cyclodextrin (HP-β-CD)
[0033] Different amounts of HP-β-CD were dissolved in 3mL PBS (pH 5.0), prepared into a series of HP-β-CD solutions with a concentration, placed in stoppered test tubes, and excess 9-NC was added to each tube, respectively at 25 ℃, 37℃, 60℃ constant temperature water bath shake for 48h, take the suspension, centrifuge at 12000rpm for 10min, take the supernatant, measure the absorbance value, and calculate the concentration. Taking the concentration of 9-NC (mmol / L) as the ordinate and the concentration of HP-β-CD (mmol / L) as the abscissa, draw the equilibrium phase solubility curves at 25°C, 37°C, and 60°C, and the results are shown in the table 1.
[0034] Table 1 Phase solubility curves at different temperatures
[0035] Table l Phase solubility curve under different temperature
[0036]
[0037] It can be seen from Table 1 that the phase solubility curves are all ...
Embodiment 2
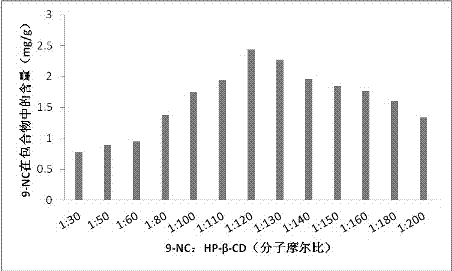
[0038] Example 2 Primary screening of 9-NC: HP-β-CD molecular molar ratio: preparation of 9-nitrocamptothecin-cyclodextrin package, the steps are as follows:
[0039] 1) 9-NC was made into 10ml of saturated acetone solution, and 13 parts were prepared, that is, 14.9mg of 9-NC in each part was dissolved in 10ml of acetone solution, and the amount of 9-NC was 0.03788mmol;
[0040] 2) According to the 9-NC:HP-β-CD molecular molar ratio of 1:30, 50, 60, 80, 100, 110, 120, 130, 140, 150, 160, 180, 200, weigh the cyclodextrin amount, and were dissolved in 5ml of water to make a cyclodextrin solution;
[0041] 3) Slowly add 13 parts of 9-NC saturated acetone solution dropwise to 13 parts of cyclodextrin solutions with different cyclodextrin concentrations, and magnetically stir at 60°C until the acetone is completely volatilized to obtain a suspension;
[0042]4) Centrifuge the prepared suspension at 2000 r.p.m for 10 min, take the supernatant and freeze-dry to obtain 9-NC-cyclodext...
Embodiment 3
[0048] Embodiment 3 Orthogonal experiments optimize the best preparation process parameters
[0049] By the result obtained in Example 2, further use the orthogonal experiment design to optimize the wrapping process, for influencing factors: the wrapping ratio (A) of medicine and HP-β-CD, the wrapping time (B) and the effect of HP-β-CD The aqueous solution concentration (C) was investigated. Take L9 (3 4 ) Orthogonal table arrangement experiment, and the packaging process optimization was carried out with the drug content of 9-NC in the finished package as the investigation index. See Table 2 for the level table of each factor, and Table 3 for the results.
[0050] Table 2 Factors and levels of orthogonal experiment
[0051] Table2Factors and levels of orthogonal test
[0052]
[0053] Table 3 Orthogonal test results
[0054] Table3 The programs and results of orthogonal test
[0055]
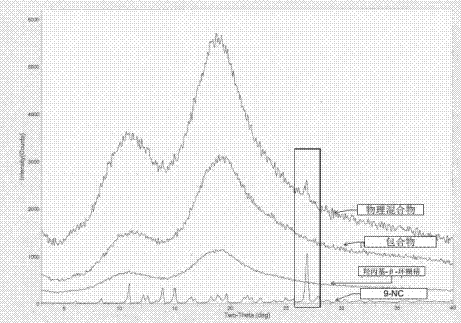
[0056] Orthogonal test results show that the best wrapping process is A 1 B 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com