Method for synthesizing 9-nitrocamptothecin
A technology of nitrocamptothecin and nitrobenzene, applied in the direction of organic chemistry, etc., to achieve the effects of small discharge of three wastes, high yield, and simple process methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of 2,6-dinitrobenzaldehyde ethylene glycol acetal
[0030] Take 2,6-dinitrobenzaldehyde (5g, 0.026mol), 20ml of ethylene glycol, and 0.15g of p-toluenesulfonic acid dissolved in 250ml of toluene, dehydrate under reflux for 5h, evaporate to dryness, add 50ml of dichloromethane and 100ml of water, Separate layers, dichloromethane layer, dry over anhydrous sodium sulfate, concentrate to dryness to obtain the product, recrystallize from methanol to obtain 5.4g, yield 88.2%, Mp (melting point) 107-109°C.
Embodiment 2
[0031] Embodiment 2: Preparation of 2-amino-6-nitrobenzaldehyde ethylene glycol acetal
[0032] Take 2,6-dinitrobenzaldehyde ethylene glycol acetal (5g, 0.02mol), sodium sulfide (10g, 0.13mol) and dissolve it in 200ml of ethanol and 50ml of water, reflux for 30min, evaporate the ethanol under reduced pressure, add dichloro Extracted with 100ml of methane, dried over anhydrous sodium sulfate, evaporated to dryness to obtain a yellow solid, recrystallized from methanol to obtain 3.9g, yield 89.1%, Mp103~105℃.
Embodiment 3
[0033] Embodiment 3: preparation 9-nitrocamptothecin
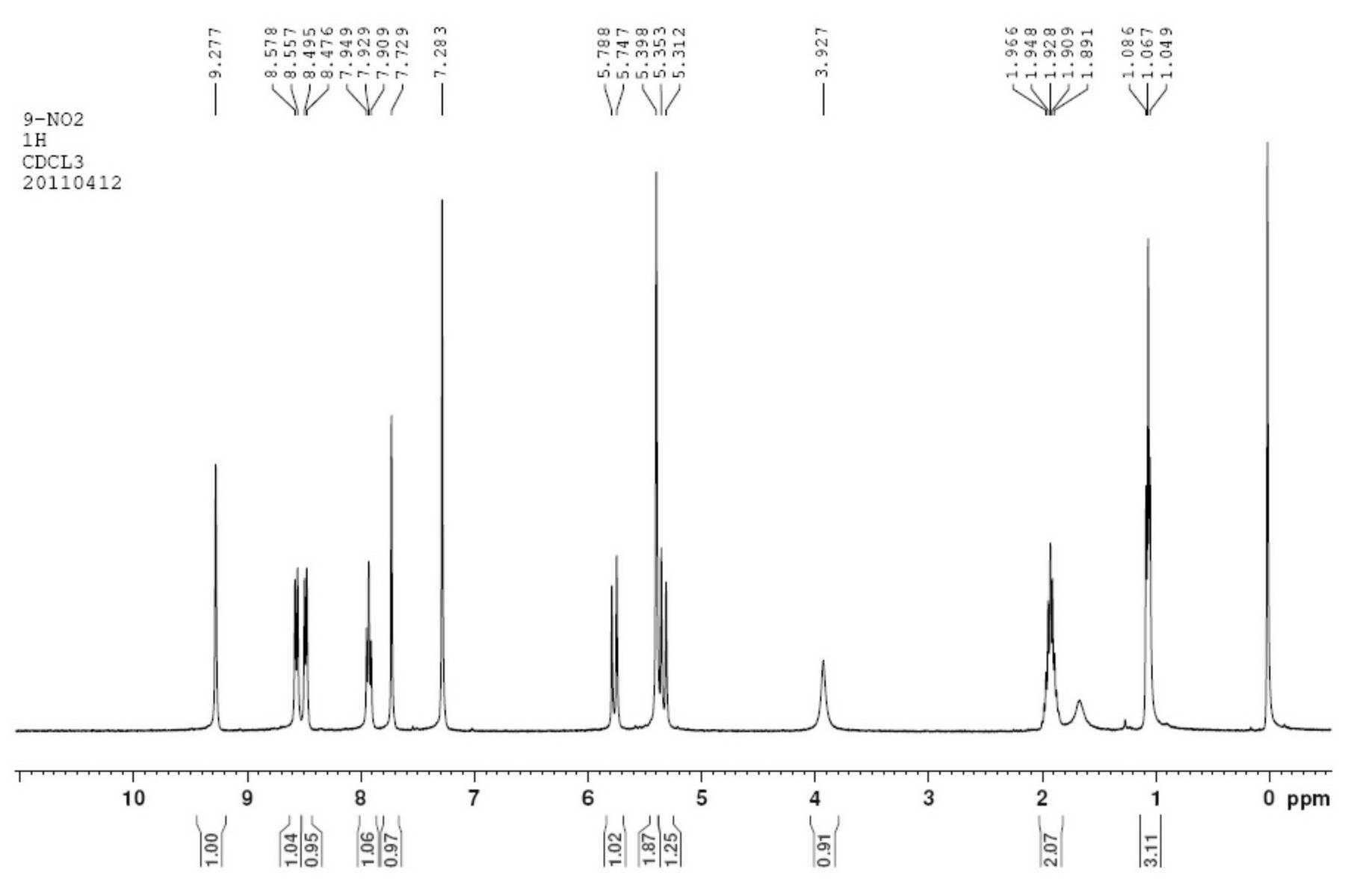
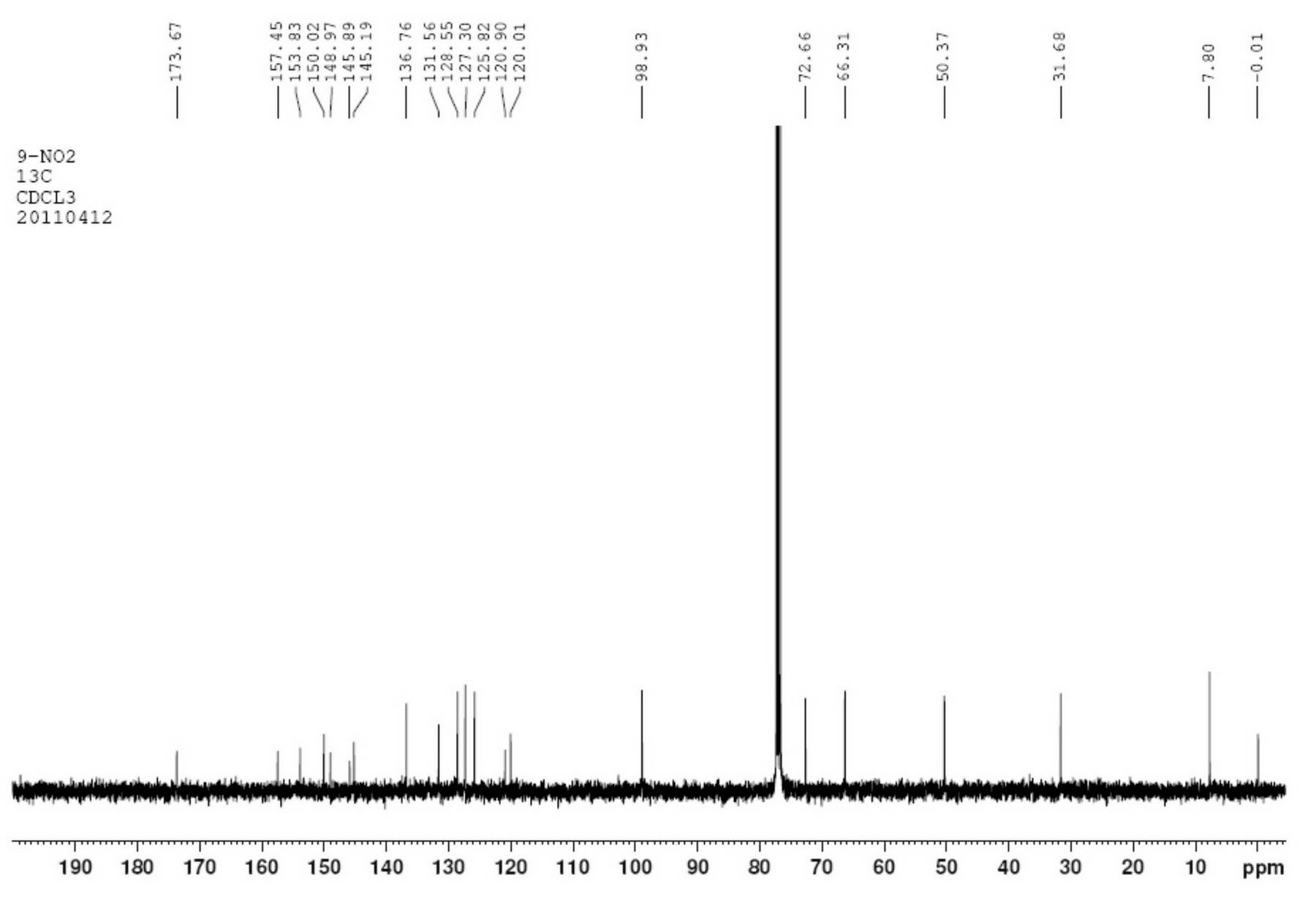
[0034] Take 2-amino-6-nitrobenzaldehyde ethylene glycol acetal (2.1g, 0.01mol) and α-(S)-tricyclic amide (3.4g, 0.008mol) in a mixed solvent of ethanol and dichloromethane Add 0.1 g of concentrated hydrochloric acid, react at 80°C for 8 hours, cool, and filter to obtain crude 9-nitrocamptothecin, which is recrystallized in ethanol to obtain 3.1 g of 9-nitrocamptothecin with a yield of 78.9%. The experimental data are as follows:
[0035] C 20 h 15 N 3 o 6 , Mp 182~186℃, ee>99% (HPLC detection conditions: chiral column OD-H; mobile phase n-hexane / isopropanol / diethylamine=6 / 4 / 0.1; flow rate 0.5ml / min; detection wavelength 254nm).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com