9-nitrocamptothecin freeze-dried powder injection and preparation method thereof
A technology of nitrocamptothecin and freeze-dried powder injection, which is applied in the direction of freeze-dried delivery, powder delivery, non-effective ingredients of polymer compounds, etc. Poor stability and other problems, to achieve the effect of improving drug safety, cost-free pollution, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
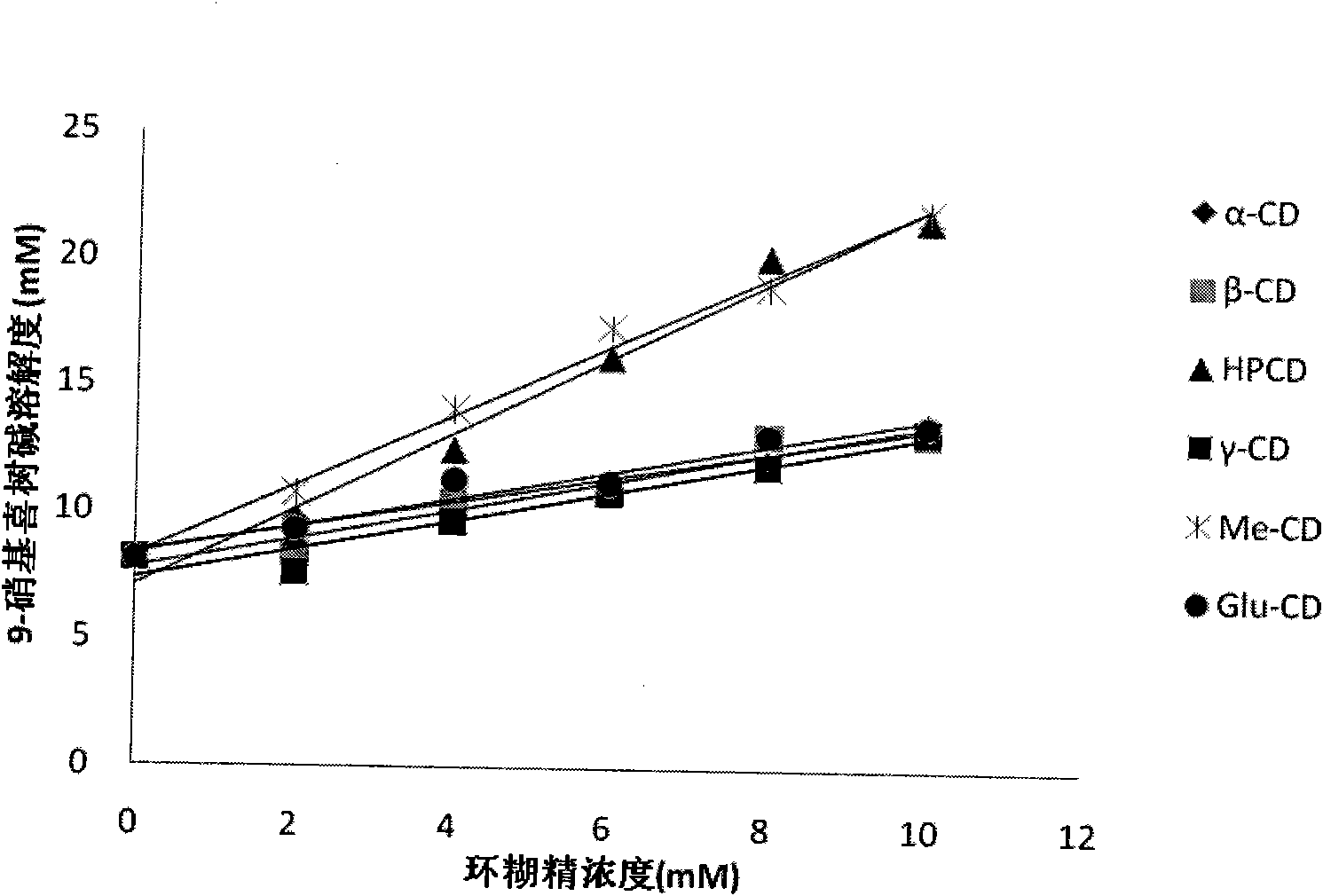
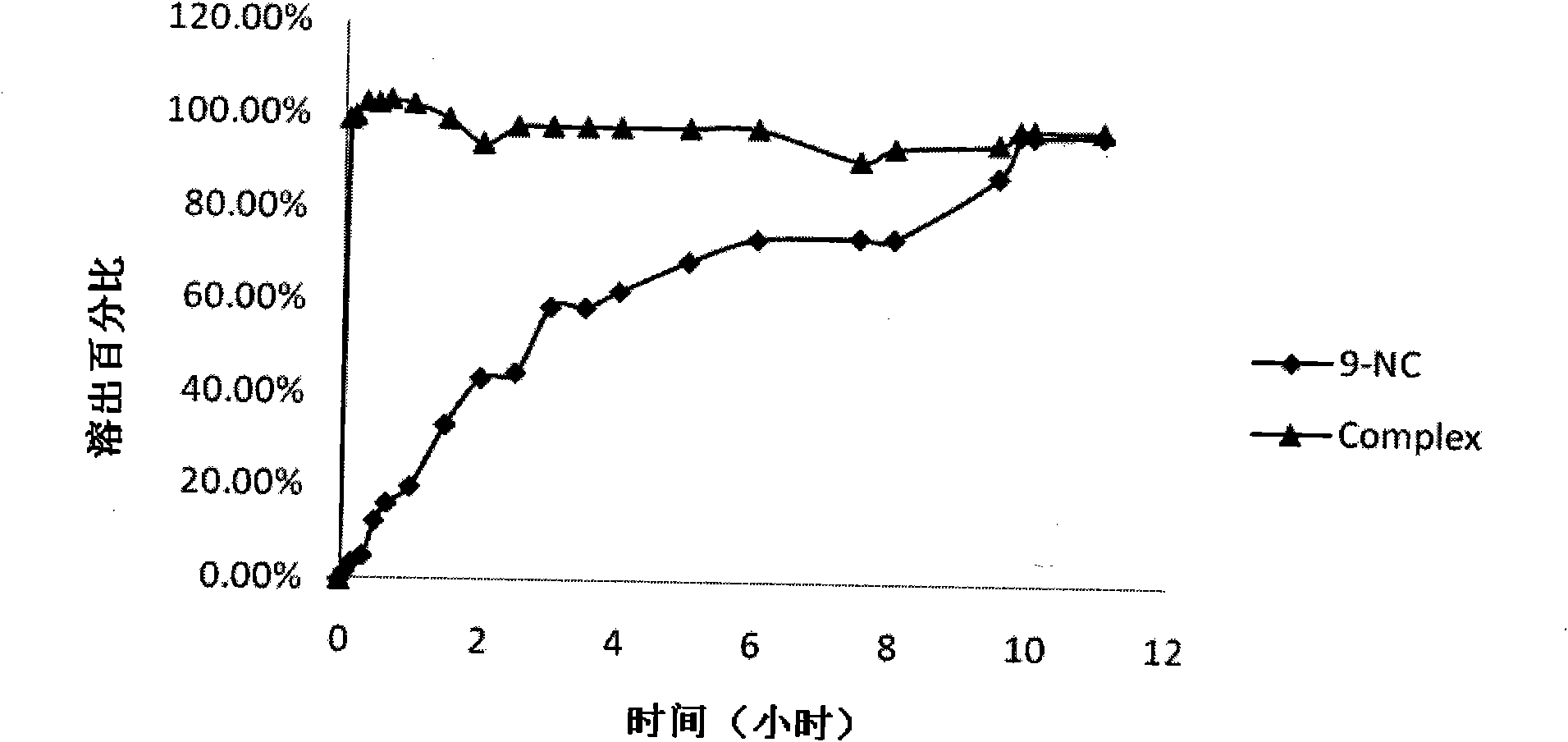
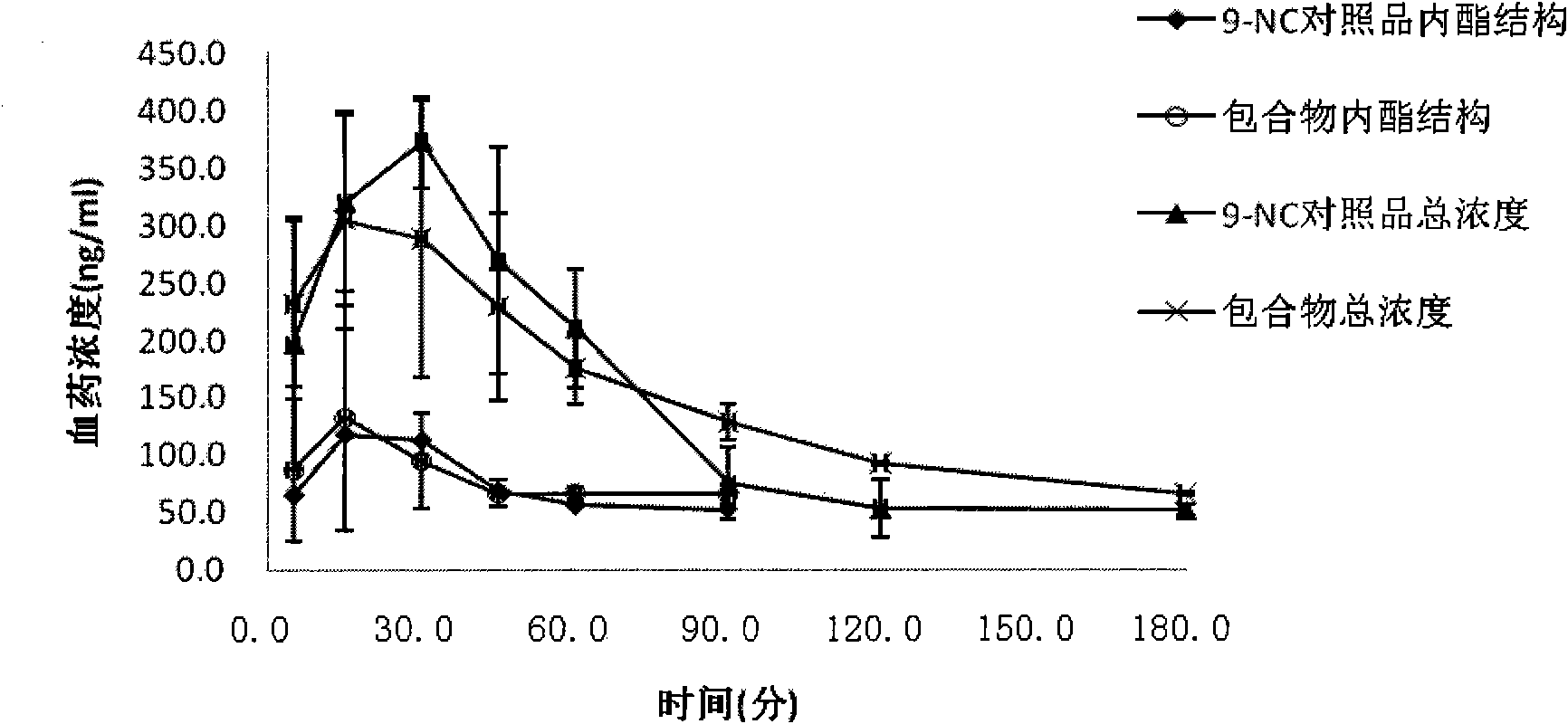
[0055] Prepare 5ml of 10mM hydroxypropyl-β-cyclodextrin aqueous solution, add 1.5mg of 9-nitrocamptothecin, stir at 50°C in the dark for 2 hours, filter, add 5% mannitol, and freeze-dry to obtain light yellow loose powder.
Embodiment 2
[0057] Prepare 5ml of 10mM glucose-β-cyclodextrin aqueous solution, add 1.5mg of 9-nitrocamptothecin, stir at room temperature in the dark for 5h, filter, add 10% glucose, and freeze-dry to obtain light yellow loose powder.
Embodiment 3
[0059] Prepare 5ml of 8mM β-cyclodextrin phosphate buffer (pH5.0), add 2ml of 9-NC saturated ethanol solution, stir for 3h at room temperature in the dark, remove ethanol by rotary evaporation, filter, add 1% dextran, and freeze-dry to obtain Light yellow loose powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com