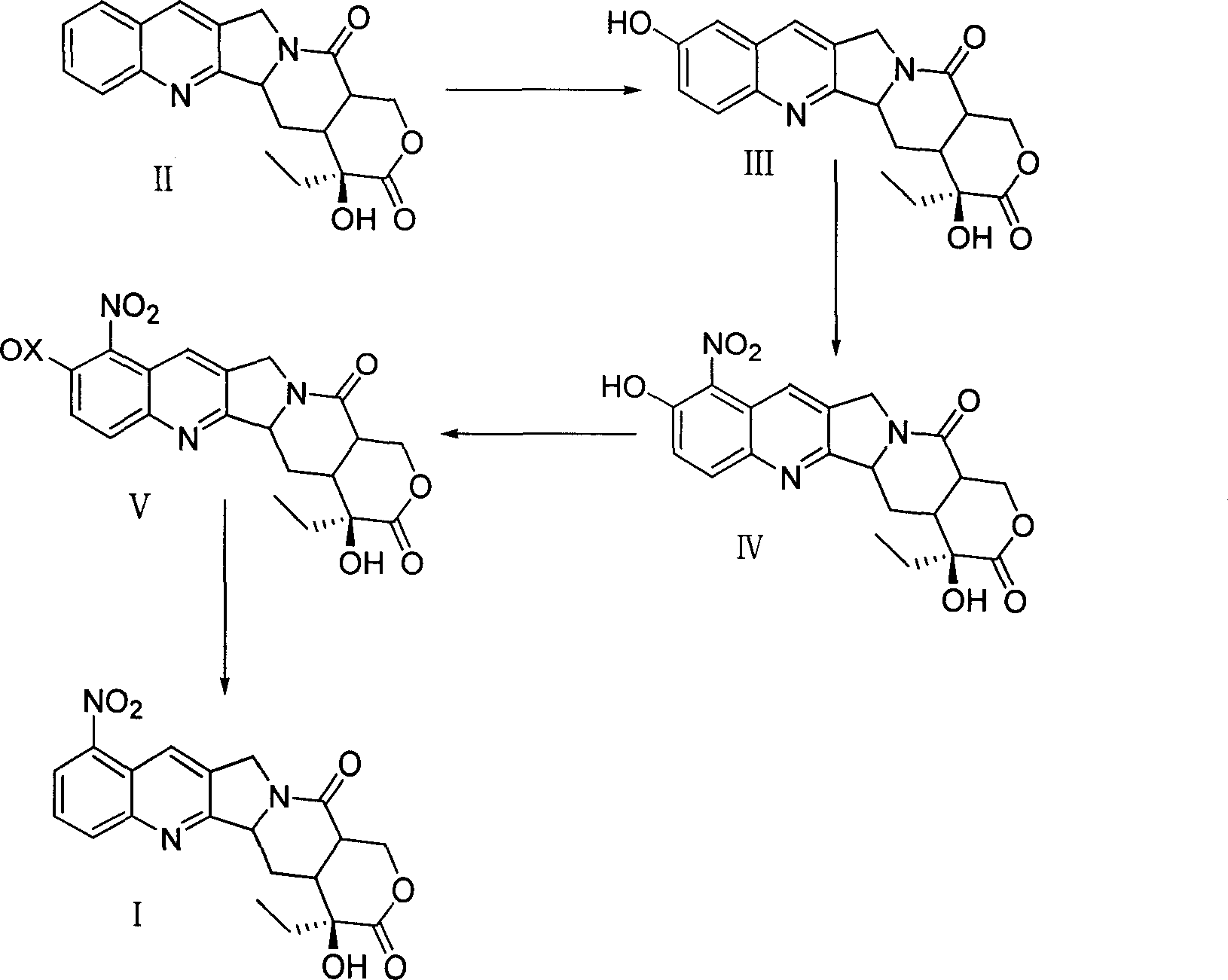
Method for preparing 9-nitro-20(s)-camptothecine
A technology of nitrocamptothecin and hydroxycamptothecin, applied in the direction of organic chemistry, which can solve the problems of low yield, difficult separation and purification, complicated separation and purification process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 10-Hydroxy-20(S)-camptothecin
[0028] 2 g of 20(S)-camptothecin, 20 ml of glacial acetic acid, 0.2 g of PtO 2 And 2 drops of dimethyl sulfoxide were added to a 100 ml autoclave. 6 kg of hydrogen pressure, 60 ° C reaction for 12 hours. After filtration, 20 ml of deionized water was added to the filtrate, and 4.8 g of iodobenzene diacetate was added in one go, and reacted at room temperature overnight. Filter and dry the solid to obtain 1.43 g of 10-hydroxy-20(S)-camptothecin with a yield of 70%.
[0029] h 1 NMR (DMSO-d 6 ), δppm: 0.86(3H, t); 1.84(2H, q); 5.21(2H, s); 5.40(2H, s); 6.54(1H, s); 7.25(1H, s); d); 7.41(1H, q); 8.00(1H, d); 8.43(1H, s); 10.42(1H, s).
Embodiment 2
[0031] 10-Hydroxy-9-nitro-20(S)-camptothecin
[0032] (Method A)
[0033] Suspend 0.365 g of 10-hydroxy-20(S)-camptothecin in 10 ml of glacial acetic acid, add dropwise 80 ul of fuming nitric acid, and react overnight at room temperature. After adding 100ml of water, a large amount of solids were precipitated, filtered and dried to obtain 0.342 g of 10-hydroxy-9-nitro-20(S)-camptothecin, yield 83.4%, HPLC, 93%.
[0034] (Method B)
[0035] Dissolve 0.365 g of 10-hydroxyl-20(S)-camptothecin in 30% dilute sulfuric acid, add 0.5 g of potassium nitrate, react at room temperature overnight, and extract with chloroform to obtain 10-hydroxyl-9-nitro-20(S) -Camptothecin 0.3 g, yield 73%.
[0036] h 1 NMR (CDCl 3 ), δppm: 1.05 (3H, t); 1.89 (2H, q); 3.74 (1H, s); 5.36 (2H, s); 5.50 (2H, dd); 7.61 (1H, s); s); 8.40(1H, d); 9.54(1H, s); 12.20(1H, s).
Embodiment 3
[0038] 9-nitro-10-(p-toluenesulfonyloxy)-20(S)-camptothecin
[0039] Suspend 0.409 g of 9-nitro-10-hydroxy-20(S)-camptothecin in 50 ml of dichloromethane, add 0.21 g of triethylamine to dissolve the solid, and add 0.2 g of p-toluenesulfonyl chloride at room temperature. React at room temperature for 24 hours, extract with dichloromethane, wash with 0.5 N hydrochloric acid and saturated sodium chloride solution, dry over anhydrous sodium sulfate, and distill off the solvent to obtain 9-nitro-10-(p-toluenesulfonyloxy)- 0.48 g of 20(S)-camptothecin, yield 83%.
[0040] h 1 NMR (CDCl 3), δppm: 1.05(3H, t); 1.90(2H, q); 2.48(3H, s); 3.87(3H, s); 5.30(2H, s); d); 7.68(1H, s); 7.81(2H, d); 7.95(1H, d); 8.31(1H, s); 8.41(1H, d).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com