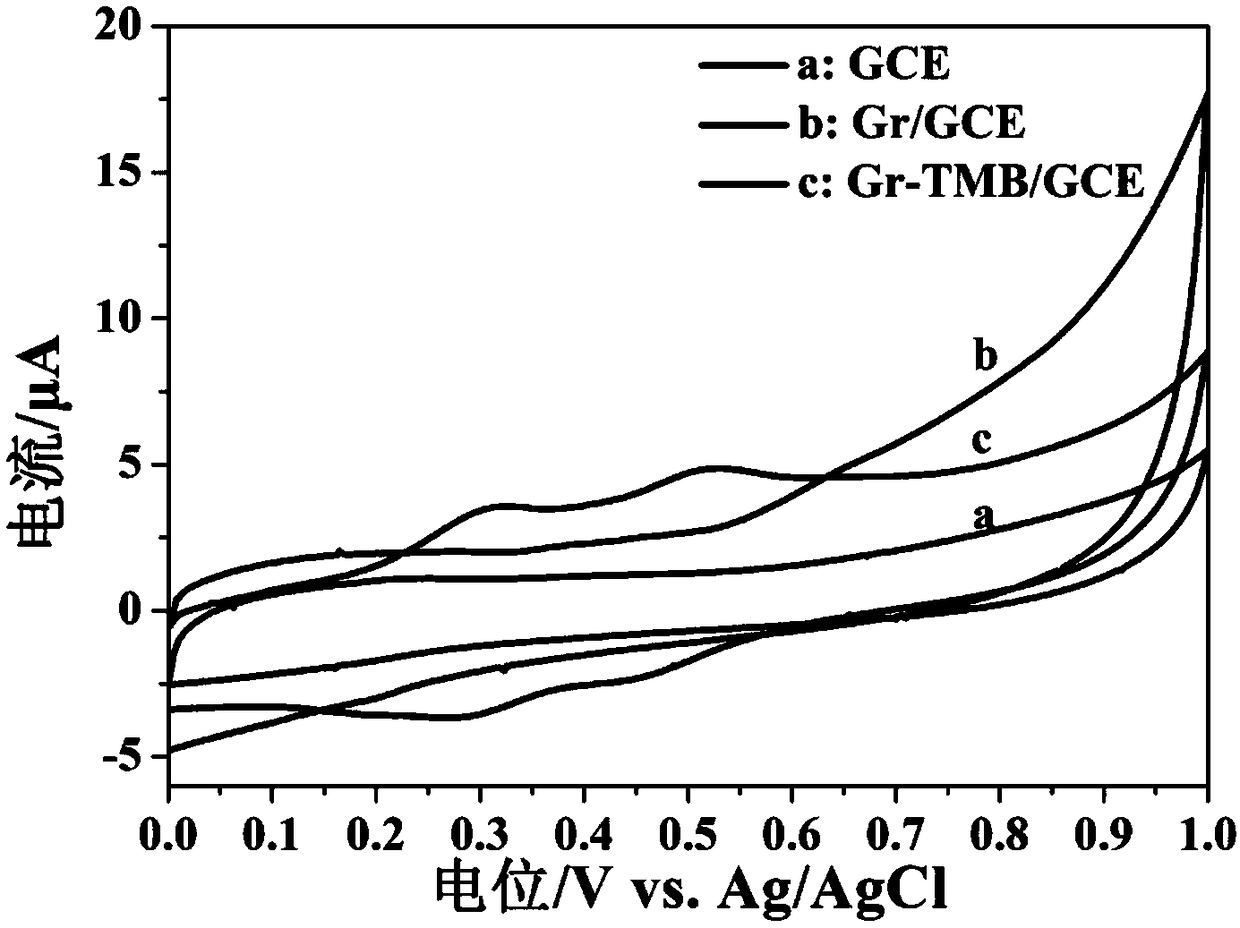
Working electrode capable of selectively detecting silver ions and electrochemical sensor
A working electrode, silver ion technology, applied in the field of electrochemical analysis, can solve the problems of non-selectivity, silver ions have no practical significance, etc., to achieve the effects of high selectivity, improved electron transfer performance, and fast response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A working electrode that can be used to selectively detect silver ions, comprising a glassy carbon electrode and a composite material modified on the surface of the glassy carbon electrode, the composite material including 3,3',5,5'-tetramethylbenzidine and graphite ene; wherein, the mass ratio of 3,3′,5,5′-tetramethylbenzidine to graphene is 1:1; the base electrode is a glassy carbon electrode.
[0047] The preparation method of above-mentioned working electrode is as follows:
[0048] 1) 3,3′,5,5′-Tetramethylbenzidine (TMB) was dissolved in N,N-dimethylformamide (DMF) to make 5mM solution A; graphene (ie Gr) Sonicate for 30 minutes, disperse in N,N-dimethylformamide (ie DMF) to make a 1mg / mL dispersion B; take 5mL of dispersion B and 5mL of solution A, mix, and place in an ice bath at 0°C Sonicate for 15 minutes, and finally centrifuge at a speed of 3000 rpm for 30 minutes to obtain a mixed solution C;
[0049] 2) Firstly, the glassy carbon electrode was polished wi...
Embodiment 2
[0052] A kind of electrochemical sensor, comprises the working electrode that embodiment 1 makes, opposite electrode and reference electrode; Wherein said opposite electrode is platinum wire, and described reference electrode is Ag / AgCl (saturated KCl solution) electrode.
[0053] Adopt above-mentioned electrochemical sensor to carry out electrochemical detection to silver ion:
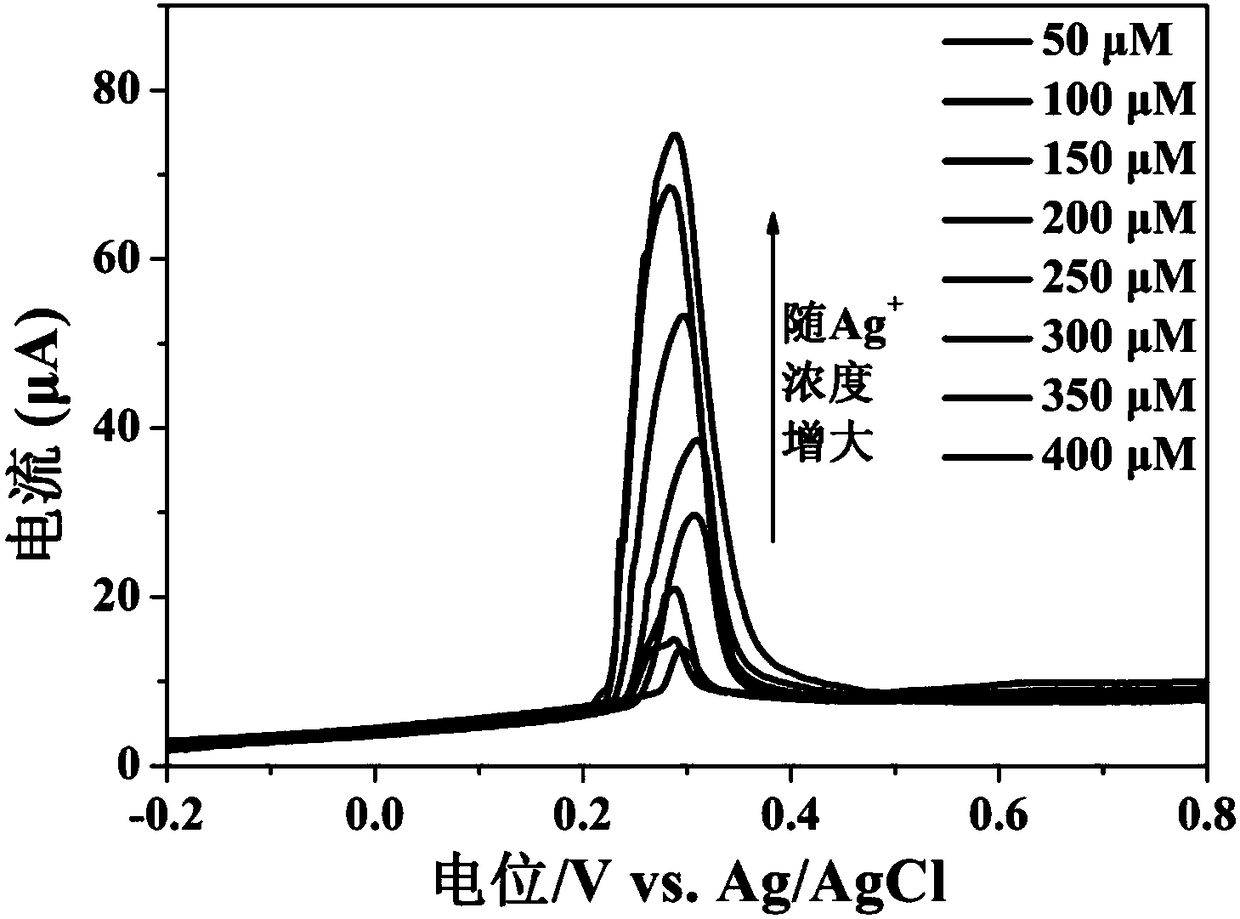
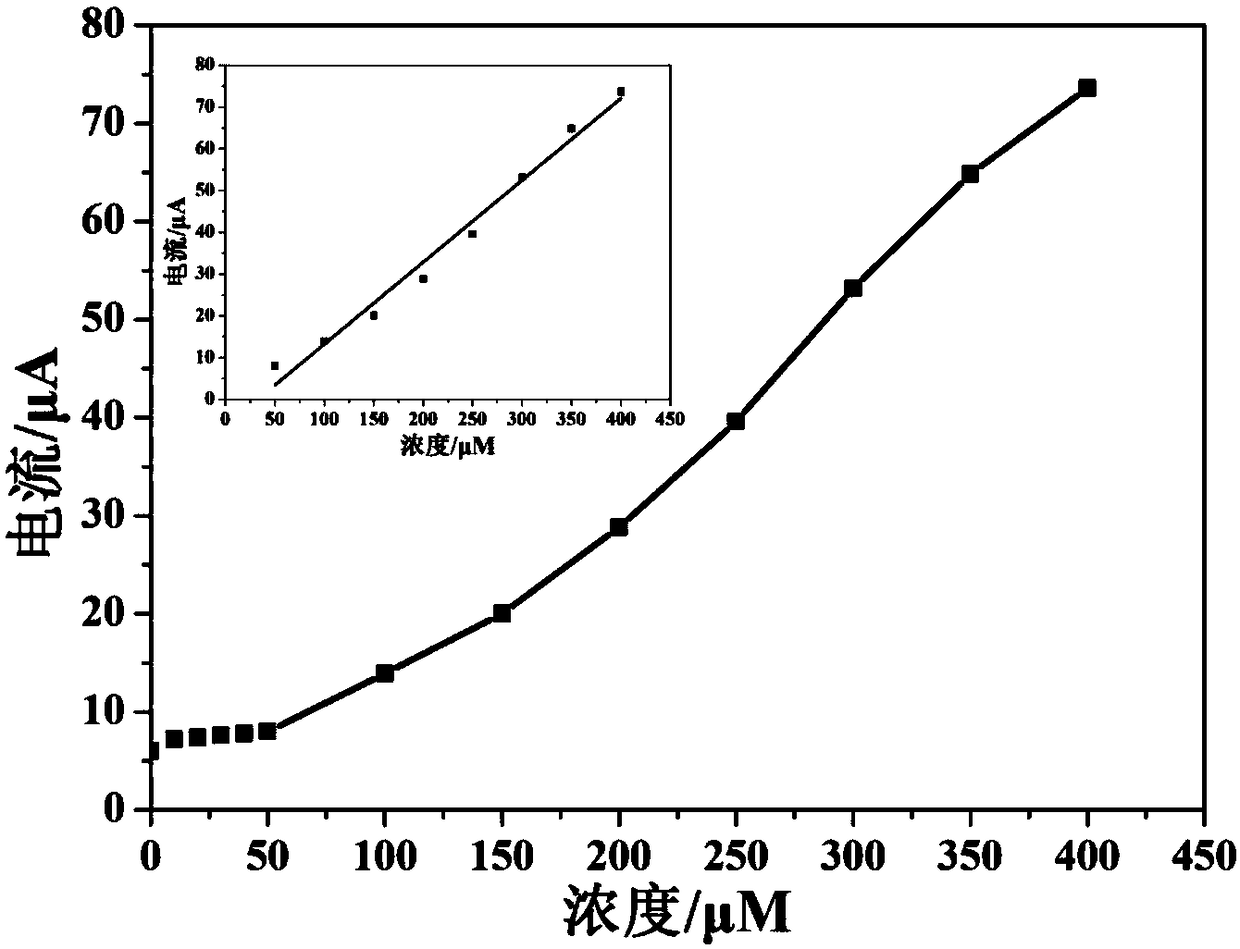
[0054] Different concentrations of silver ions (i.e. Ag + ) into the sodium acetate buffer solution with a pH of 4.0, using the working electrode prepared in Example 1, utilizing differential pulse voltammetry to silver ions (i.e. Ag + ) for measurement, the linear increase of the peak current indicates that the sensing electrode can successfully detect silver ions of unknown concentration (i.e. Ag + ).
[0055] figure 2 and image 3 Respectively show that the electrochemical sensor of this embodiment detects different concentrations of silver ions (i.e. Ag + ) differential pulse voltammetry cur...
Embodiment 3
[0061] Detect the influence of the volume ratio of the solution A containing 3,3',5,5'-tetramethylbenzidine and the solution B containing graphene on the electrochemical sensor, that is, the preparation method of the working electrode is the same as in Example 1, except that The point is only to change the volume ratio of solution A and solution B.
[0062] Adopt the electrochemical sensor that comprises above-mentioned working electrode to carry out electrochemical detection to the silver ion that concentration is 200 μ M, wherein electrochemical sensor also comprises counter electrode and reference electrode; Wherein said counter electrode is platinum wire, and described reference electrode is Ag / AgCl (saturated KCl solution) electrode.
[0063] Detection method is with embodiment 2, and result is as shown in the following table:
[0064] Table 2. Relationship between volume ratio and peak current value
[0065] The volume ratio of solution A and solution B
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com