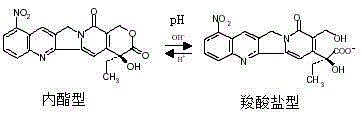
9-nitrocamptothecin-cyclodextrin inclusion compound, its preparation method and pharmaceutical composition containing the inclusion compound
A technology of cyclodextrin inclusion complex and nitrocamptothecin, which can be used in drug combinations, medical preparations containing active ingredients, antitumor drugs, etc., can solve problems such as low bioavailability and poor solubility of 9-NC , to achieve the effect of improving dissolution, solubility and significant sustained release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19-N
[0032] Phase solubility curve experiment of embodiment 19-NC and hydroxypropyl-β-cyclodextrin (HP-β-CD)
[0033] Different amounts of HP-β-CD were dissolved in 3mL PBS (pH 5.0), prepared into a series of HP-β-CD solutions with a concentration, placed in stoppered test tubes, and excess 9-NC was added to each tube, respectively at 25 ℃, 37℃, 60℃ constant temperature water bath shake for 48h, take the suspension, centrifuge at 12000rpm for 10min, take the supernatant, measure the absorbance value, and calculate the concentration. Taking the concentration of 9-NC (mmol / L) as the ordinate and the concentration of HP-β-CD (mmol / L) as the abscissa, draw the equilibrium phase solubility curves at 25°C, 37°C, and 60°C, and the results are shown in the table 1.
[0034] Table 1 Phase solubility curves at different temperatures
[0035] Table 1Phasesolubilitycurveunderdifferenttemperature
[0036]
[0037] It can be seen from Table 1 that the phase solubility curves are all linear...
Embodiment 2
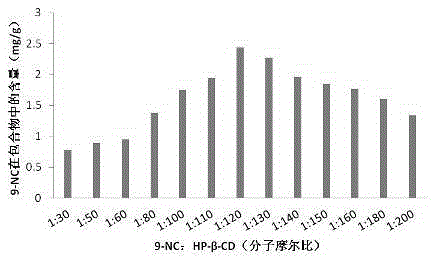
[0038] Example 2 Primary screening of 9-NC: HP-β-CD molecular molar ratio: preparation of 9-nitrocamptothecin-cyclodextrin inclusion compound, the steps are as follows:
[0039] 1) 9-NC was made into 10ml of saturated acetone solution, and 13 parts were prepared, that is, 14.9mg of 9-NC in each part was dissolved in 10ml of acetone solution, and the amount of 9-NC was 0.03788mmol;
[0040] 2) According to the 9-NC:HP-β-CD molecular molar ratio of 1:30, 50, 60, 80, 100, 110, 120, 130, 140, 150, 160, 180, 200, weigh the cyclodextrin amount, and were dissolved in 5ml of water to make a cyclodextrin solution;
[0041] 3) Slowly add 13 parts of 9-NC saturated acetone solution dropwise to 13 parts of cyclodextrin solutions with different cyclodextrin concentrations, and magnetically stir at 60°C until the acetone is completely volatilized to obtain a suspension;
[0042] 4) Centrifuge the prepared suspension at 2000 r.p.m for 10 min, take the supernatant and freeze-dry to obtain 9-...
Embodiment 3
[0048] Embodiment 3 Orthogonal experiments optimize the best preparation process parameters
[0049] By the results obtained in Example 2, further use the orthogonal experiment design to optimize the inclusion process, to influence factors: inclusion ratio (A) of medicine and HP-β-CD, inclusion time (B) and HP-β-CD The aqueous solution concentration (C) was investigated. With L9(3 4 ) Orthogonal table arrangement experiment, the inclusion process is optimized with the drug content of 9-NC in the finished inclusion compound as the investigation index. See Table 2 for the level table of each factor, and Table 3 for the results.
[0050] Table 2 Factors and levels of orthogonal experiment
[0051] Table 2 Factors and levels so for thogonal test
[0052]
[0053] Table 3 Orthogonal test results
[0054] Table 3 The programs and results so for thogonal test
[0055]
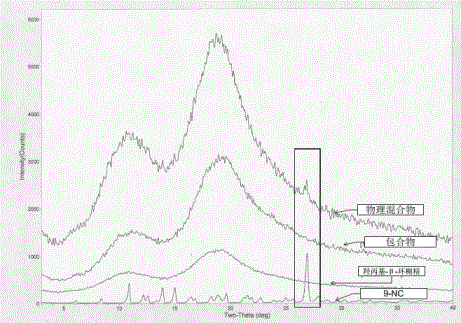
[0056] Orthogonal test results show that the best inclusion process is A 1 B 2 C 3 , ie drug: HP-β-C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com