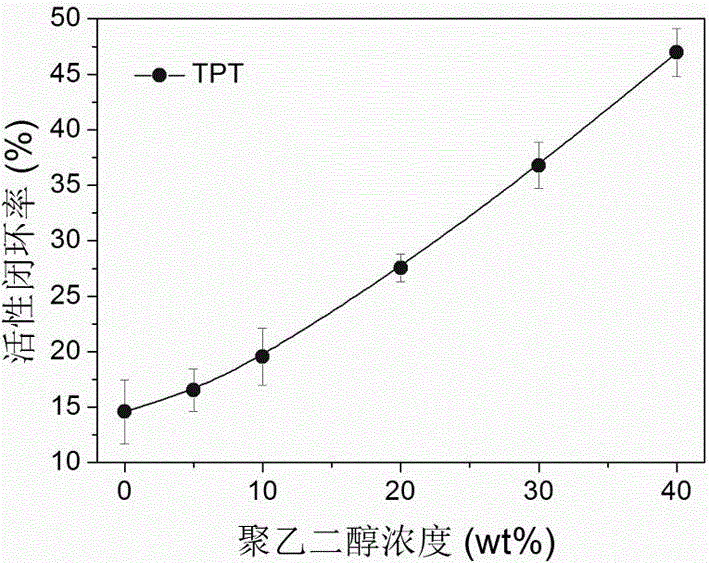
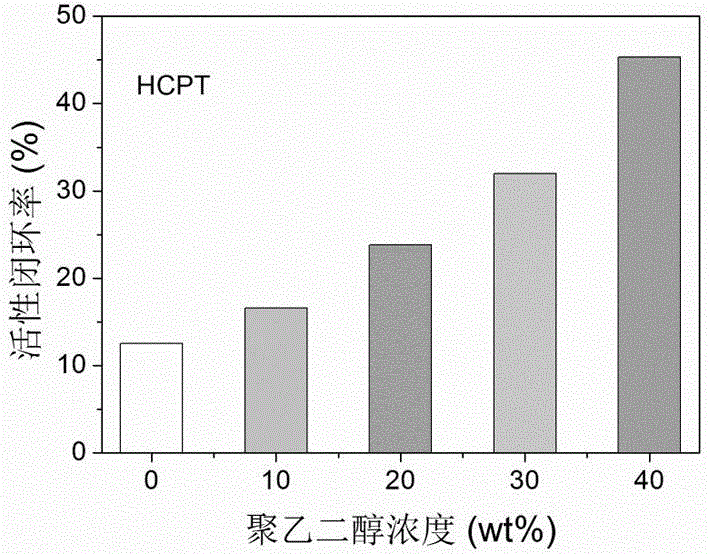
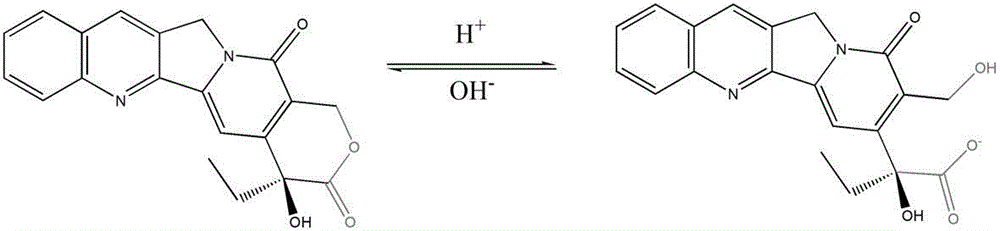Liquid preparation for improving active ring-closing rate of camptothecin compounds and its preparation method and application
A technology of liquid preparations and camptothecin, which is applied in the field of medicine and can solve problems such as changing the ratio of active ingredients of drug molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0039] Examples 1-9: Different end groups of polyethylene glycol
Embodiment 1
[0041] Take 15g of polyethylene glycol 1500 (PEG1500), add 5g of succinic anhydride, 2ml of pyridine, dissolve in 200ml of dichloromethane, and reflux for 48 hours. After removing part of the organic solvent and pyridine by suspension evaporation, unreacted succinic anhydride was precipitated by cooling, and the filtrate was filtered off. Rotary evaporation again to remove most of the organic solvent, precipitation with 10 times excess diethyl ether, filtration to obtain the product, vacuum drying to obtain a carrier material with carboxyl groups at both ends.
Embodiment 2
[0043] Take 20g of monomethoxypolyethylene glycol 2000 (PEG2000), add 5g of succinic anhydride, 2ml of pyridine, dissolve in 200ml of dichloromethane, and reflux for 48 hours. After removing part of the organic solvent and pyridine by suspension evaporation, unreacted succinic anhydride was precipitated by cooling, and the filtrate was filtered off. Rotary evaporation again to remove most of the organic solvent, precipitation with 10 times excess ether, filtration to obtain the product, and vacuum drying to obtain a carrier material with a single terminal group of carboxyl group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com