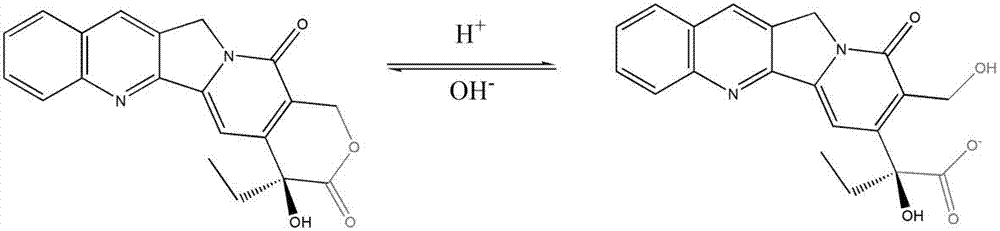
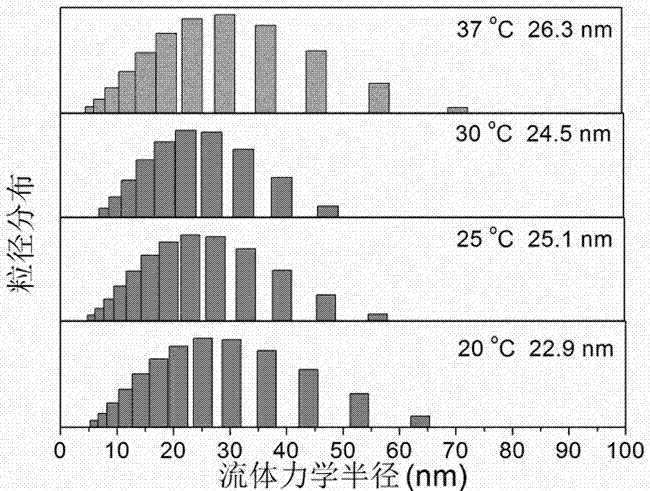
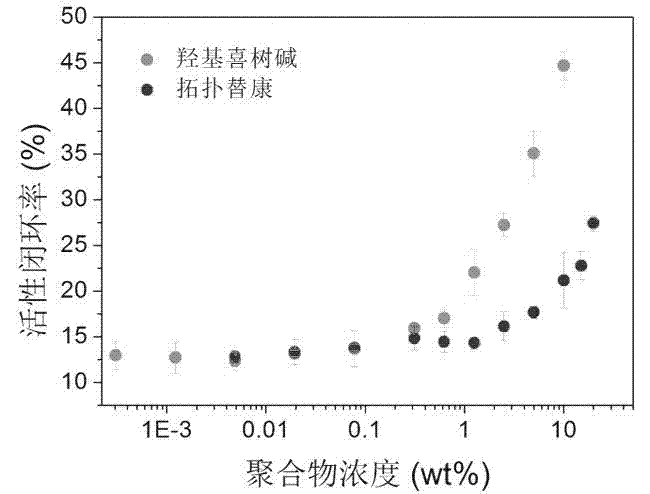
Liquid micellar preparation for increasing active closed-loop rate of camptothecin derivatives as well as preparation method and applications of preparation
A technology of liquid glue and camptothecin, which is applied in the direction of drug combination, emulsion delivery, anti-tumor drugs, etc., can solve the problems of loss of anti-tumor activity, structural instability, toxic and side effects, etc., to improve the active closed-loop rate, enhance Drug efficacy, effect of reducing systemic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0053] Examples 1-10: Synthesis of different polymeric support materials
Embodiment 1
[0055] Add 30g of dihydroxypolyethylene glycol (1500) into a 500ml three-necked flask, heat the oil bath to 130°C to remove water under vacuum for 4 hours, cool to 70°C with argon, add 59g of lactide, 7g of glycolide, 26 mg of stannous octoate (containing a small amount of toluene), evacuated at 100°C for 30 minutes, blown with argon, and heated to 140°C for 12 hours. After the reaction was completed, the product was poured out while it was hot. After cooling, the product was dissolved in dichloromethane and ether precipitated. The PLGA-PEG-PLGA micellar carrier material is obtained. The material was tested by H 1 NMR detection molecular weight is 1670-1500-1670, GPC detection molecular weight distribution coefficient ( M w / M n ) is 1.23.
Embodiment 2
[0057] Add 30g of monomethoxy-capped PEG (MPEG, 1500) into a 500ml three-necked flask, remove water under vacuum at 130°C for 4 hours, cool to 70°C with argon, add 31g of lactide, 7.5g of glycolide, 13 mg of stannous octoate (containing a small amount of toluene), evacuated at 100 °C for 30 minutes, passed argon, and heated to 130 °C for 12 hours. After the reaction is completed, pour the product into hot water at 80°C while it is still hot, and discard the upper part of the water after fully settling to obtain an MPEG-PLGA carrier material. The molecular weight detected by NMR is 1500-1820, and the molecular weight distribution coefficient detected by GPC ( M w / M n ) is 1.28.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com