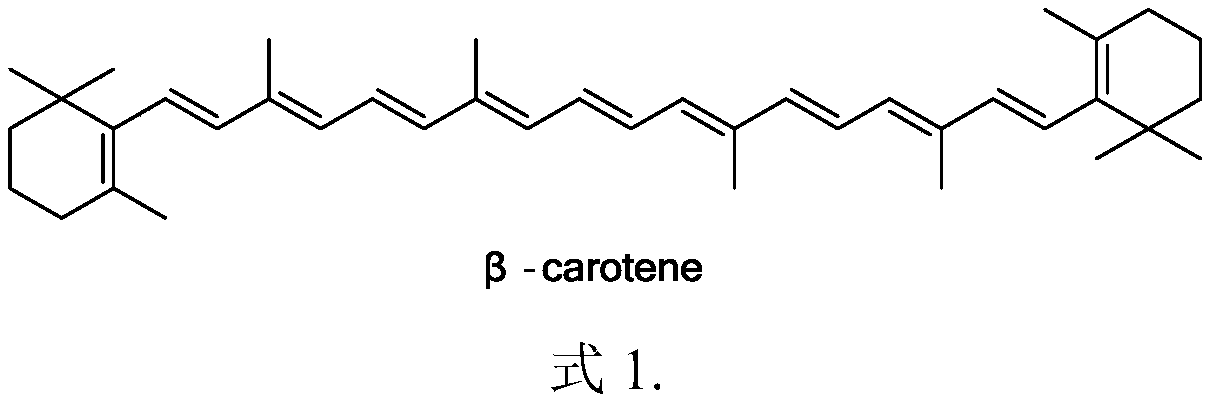
Method for preparing all-trans-beta-carotene
A carotene, all-trans technology, which is applied in the directions of organic chemistry methods, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc. problems, to achieve the effect of inhibiting the occurrence of side reactions, reducing solubility, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] 55.13g (0.11mol) of 2,4-pentadiene pentadecyl phosphate, 8.21g (0.05mol) of 2,7-dimethyl-2,4,6-octatriene-1,8- Dialdehyde, 12.8g (0.12mol) sodium carbonate, 150g anhydrous methanol are stirred and dissolved under a nitrogen atmosphere;
[0071] Mix 0.11g (0.5mmol) No.1 ionic liquid with 10g methanol, add the methanol solution of the ionic liquid dropwise into the reaction system at 10°C for 20min, and continue the reaction at 10°C for 3h after the dropwise addition;
[0072] After the reaction was completed, it was filtered, washed with water, and dried to obtain 25.46 g of all-trans β-carotene with a yield of 95%, and the content determined by HPLC was 97%.
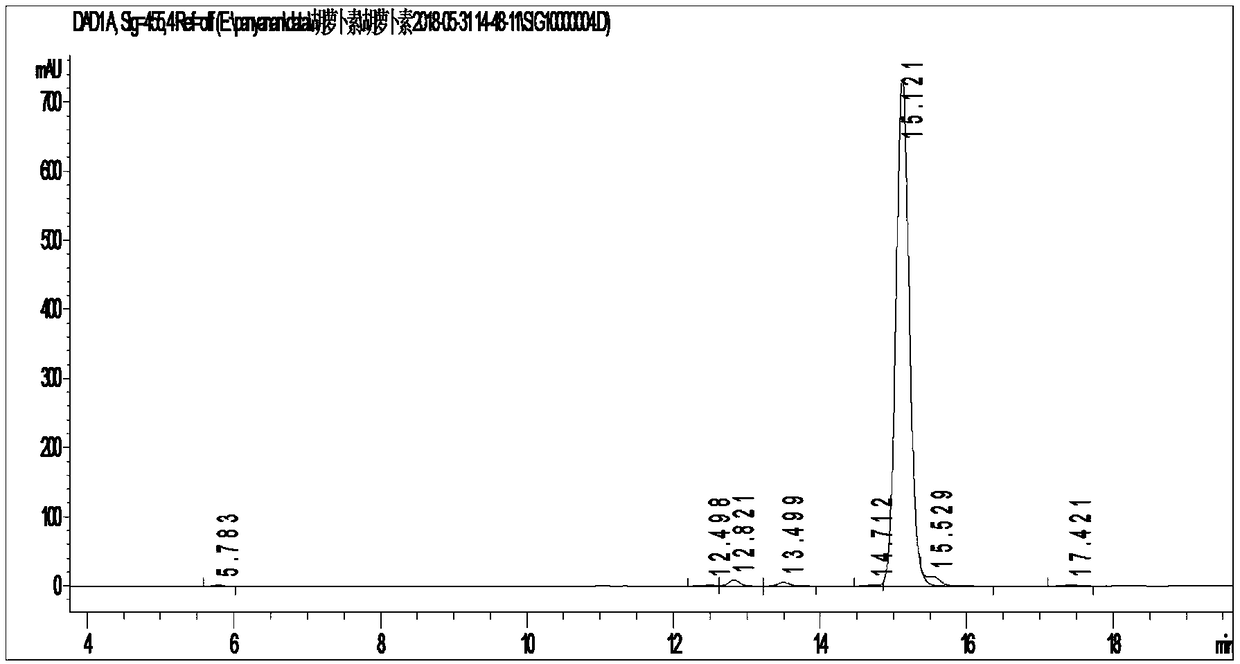
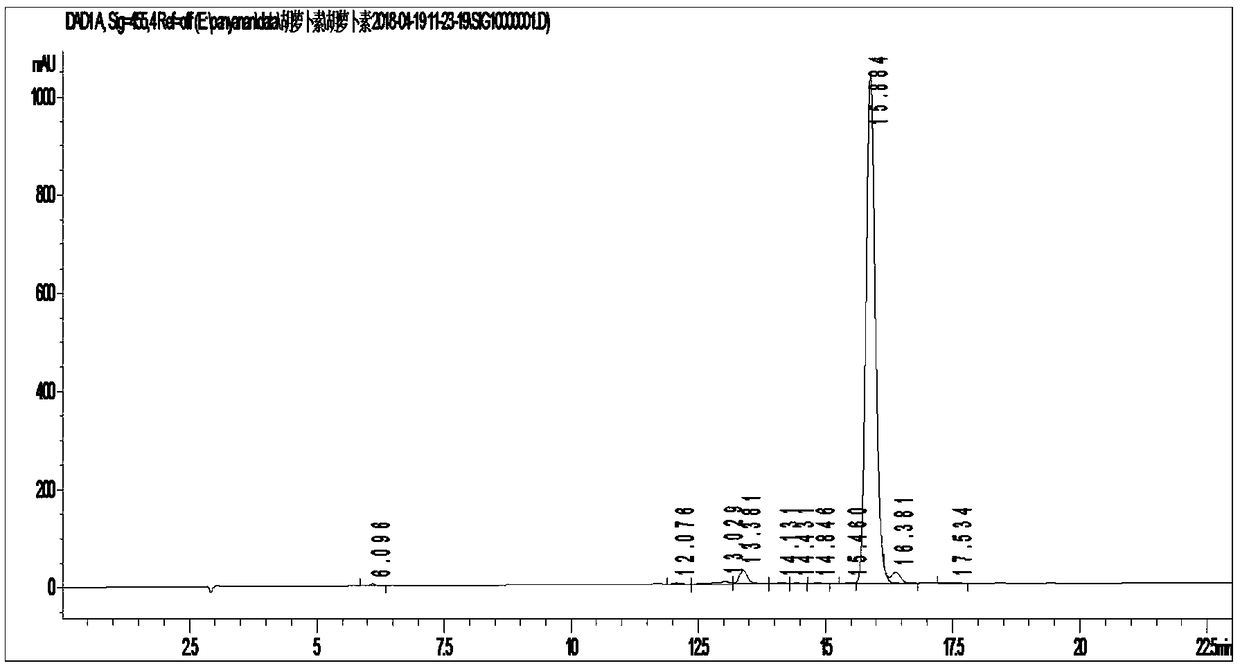
[0073] figure 1 For trans-β-carotene standard high performance liquid chromatography (purchased from Zhejiang Xinhecheng Pharmaceutical Co., Ltd.), figure 2 It is the high performance liquid phase spectrum of trans-β-carotene synthesized by the present invention.
[0074] According to HPLC-MC identification, ...
Embodiment 2
[0076] 55.13g (0.11mol) of 2,4-pentadiene pentadecyl phosphate, 8.21g (0.05mol) of 2,7-dimethyl-2,4,6-octatriene-1,8- Dialdehyde, 12.8g (0.12mol) sodium carbonate, 150g anhydrous methanol are stirred and dissolved under a nitrogen atmosphere;
[0077] Mix 0.11g (0.5mmol) No.1 ionic liquid with 10g methanol, add the methanol solution of the ionic liquid into the reaction system dropwise at -10°C, dropwise for 20min, and continue the reaction at -10°C for 3h after the dropwise addition ;
[0078] After the reaction was completed, it was filtered, washed with water, and dried to obtain 23.3 g of all-trans β-carotene with a yield of 87%, and the content determined by HPLC was 97%.
Embodiment 3
[0080] 55.13g (0.11mol) of 2,4-pentadiene pentadecyl phosphate, 8.21g (0.05mol) of 2,7-dimethyl-2,4,6-octatriene-1,8- Dialdehyde, 12.8g (0.12mol) sodium carbonate, 150g anhydrous methanol are stirred and dissolved under a nitrogen atmosphere;
[0081] Mix 0.11g (0.5mmol) of No.1 ionic liquid with 10g of methanol, add the methanol solution of the ionic liquid into the reaction system dropwise at 60°C for 20min, and continue the reaction at 60°C for 3h after the addition;
[0082] After the reaction was completed, it was filtered, washed with water, and dried to obtain 21.98 g of all-trans β-carotene with a yield of 82%, and the content determined by HPLC was 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com