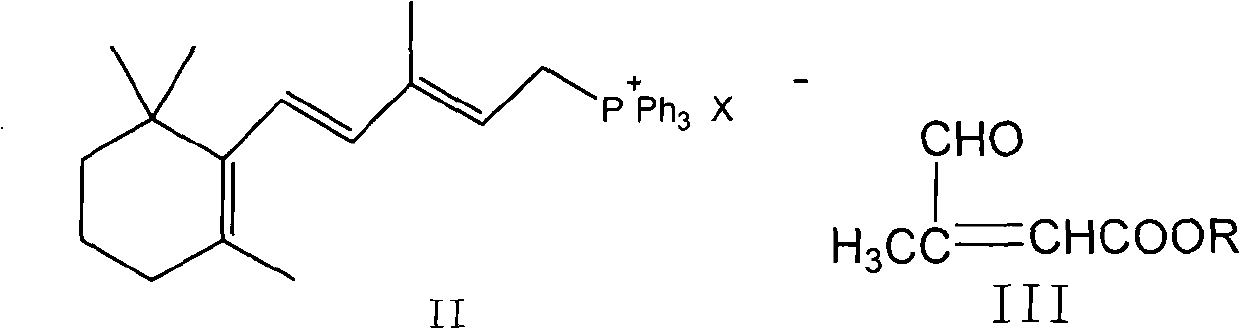
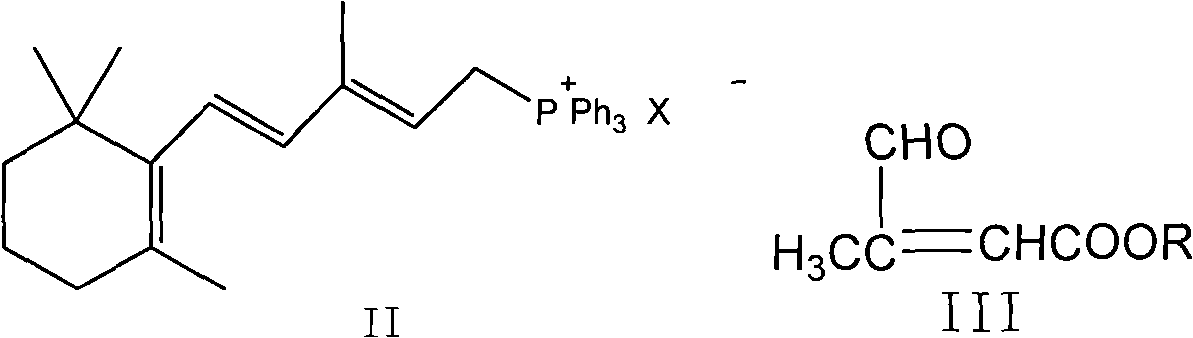
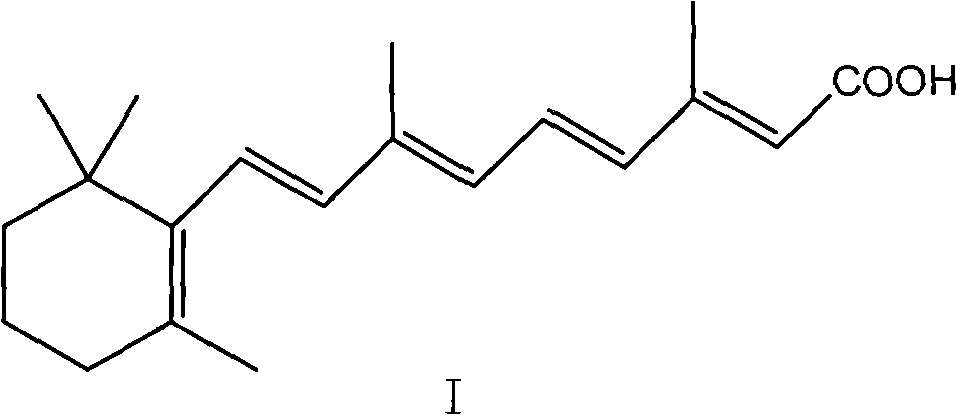
Method for preparing all-trans tretinoin
A technology of all-trans retinoic acid and retinoic acid, applied in the field of preparation of all-trans retinoic acid, can solve the problems of low yield of all-trans retinoic acid, high cost, difficult operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of the mixture of embodiment 1 all-trans retinoic acid (I) and 11-cis-retinoic acid (VI)
[0039] Under nitrogen conditions, 500.4g of [3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadiene]-triphenyl Phosphine chloride salt (WITTIG salt) and 187g of β-formyl butyl crotonate were dissolved in 1947ml of isopropanol, cooled to -10°C, 1250ml of 2N potassium hydroxide isopropanol solution was added dropwise, and When the temperature was kept between -10 and 0°C, the addition was completed and reacted for 1 hour. The reaction solution was added to water, extracted with 2×1000ml of petroleum ether, the organic layers were combined, washed with 2×300ml of water, and evaporated under reduced pressure. The solvent was removed to obtain 325 g (yield 99.0%) of a brown oily liquid, which contained 61% all-trans butyl retinoate and 36.1% 11-cis-butyl retinoate as detected by HPLC.
[0040] Under the condition of blowing nitrogen, 325 g of the mixture of all-...
Embodiment 211
[0041] Example 211 - Transformation of cis-retinoic acid into all-trans retinoic acid (1)
[0042] Under the condition of nitrogen flow, the mixed acid solution prepared in Example 1 was heated to 50° C., then a mixture of 330 mg palladium nitrate, 2160 mg triphenylphosphine, 1600 mg triethylamine and 250 ml acetonitrile was added, and the mixture was reacted for 1 hour and detected by HPLC The result was 95.3% all-trans retinoic acid and 1.1% 11-cis-retinoic acid, 600-700g of solvent was evaporated under reduced pressure, cooled to 0°C, kept for 2 hours, filtered to obtain 226.2g of all-trans retinoic acid, detected by HPLC The content is 99.6%.
Embodiment 311
[0043] Example 311-Transformation of cis-retinoic acid to all-trans retinoic acid (2)
[0044] Under the condition of nitrogen flow, the mixed acid solution prepared in Example 1 was heated to 50° C., then a mixture of 116 mg of palladium nitrate, 796 mg of triphenylphosphine, 528 mg of triethylamine and 132 ml of acetonitrile was added, reacted for 2 hours, and detected by HPLC The result was 93.3% all-trans retinoic acid and 3.1% 11-cis-retinoic acid, 600-700g of solvent was evaporated under reduced pressure, cooled to 0°C, kept for 2 hours, filtered to obtain 214.2g of all-trans retinoic acid, detected by HPLC The content is 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com