Non-spherical drug-loaded particles and controlled release preparation of lactyl polymer and preparation methods thereof
A technology of drug-loaded particles and sustained-release preparations, which is applied in the direction of active ingredients of hydroxyl compounds, pharmaceutical formulations, and medical preparations of non-active ingredients, etc., to achieve the effects of mature technology, no immunogenicity, and good reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
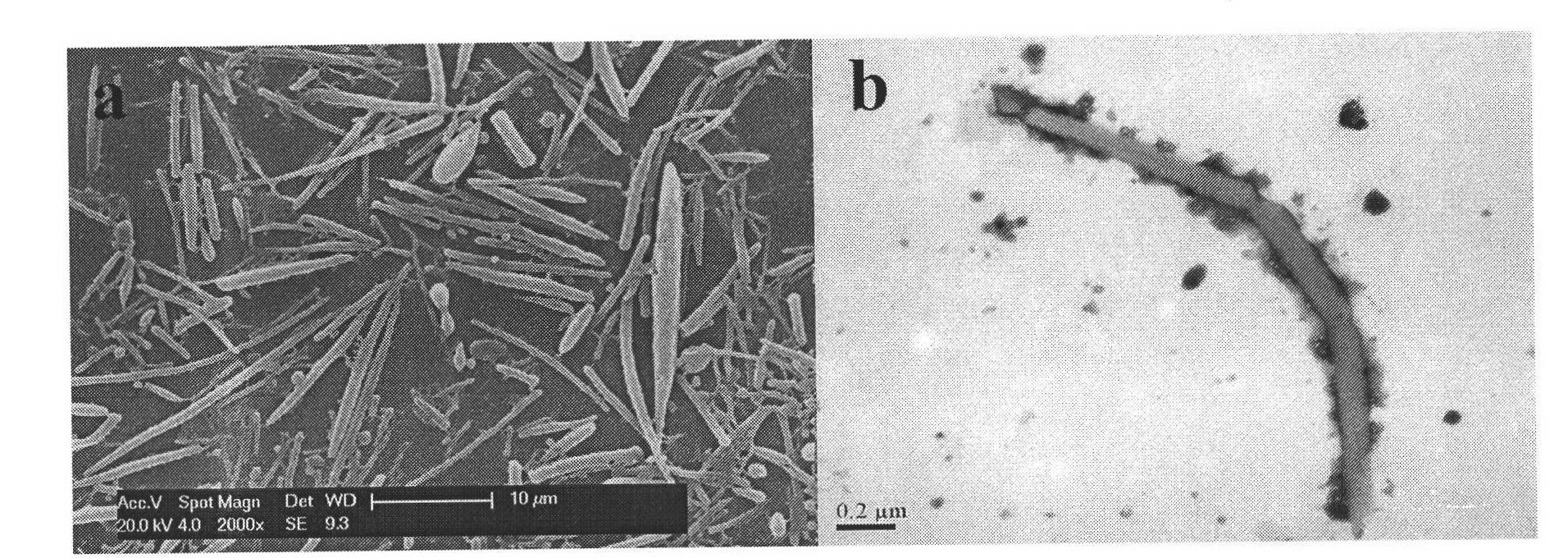
[0031] Weigh 150 mg of polylactic-co-glycolic acid (PLGA) (molecular weight: 10,000) and add it to 15 mL of dichloromethane, stir until dissolved at room temperature; 5 P 3 o 10 , content 90-95%) was added to 0.5% PVA (polymerization degree 550-650, alcoholysis degree 88%) solution and stirred until dissolved. Mix 15mL PLGA dichloromethane solution with 100mL PVA solution containing STP, stir at 1200rpm for 2 hours, centrifuge at 10000rpm, wash off the surfactant with deionized water, and freeze-dry at -30°C to obtain a white powder. The yield was 97%. The SEM pictures of the particles are shown in figure 1 , the shape is rod-like, the length is 6-9 μm, the diameter is 0.5-1.6 μm, the aspect ratio is 5-12, the physical and chemical properties of the particles are stable, and the reproducibility is good.
Embodiment 2
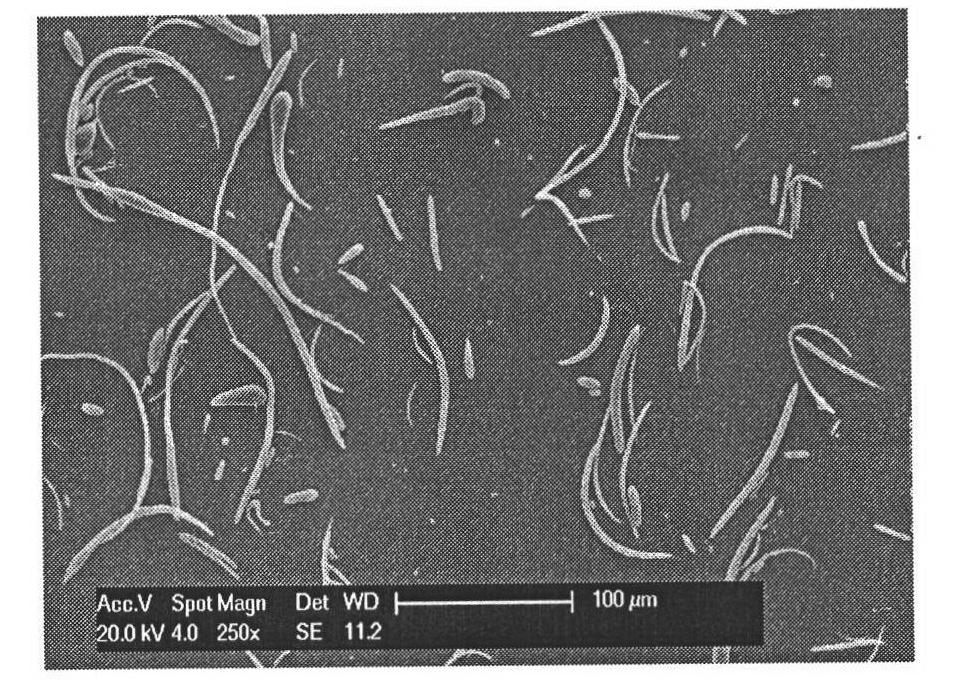
[0033] Weigh 100 mg of polylactic-co-glycolic acid (PLGA) (molecular weight: 50,000) and add it to 10 mL of dichloromethane, stir until dissolved at room temperature; add 0.735 g of sodium polyphosphate (STP) to 0.4% PVA (polymerized degree 550~650, degree of alcoholysis 88%) and stirred in the solution until dissolved. Mix 10mL PLGA dichloromethane solution with 100mL PVA solution containing STP, stir at 1200rpm for 2 hours, centrifuge at 10000rpm, wash the surfactant with deionized water, and freeze-dry at -30°C to obtain a white powder. The yield was 86%. The SEM pictures of the particles are shown in figure 2 , the shape is fibrous, the length is 18-50 μm, the diameter is 0.4-5 μm, the aspect ratio is 6-14, the physical and chemical properties of the particles are stable, and the reproducibility is good.
Embodiment 3
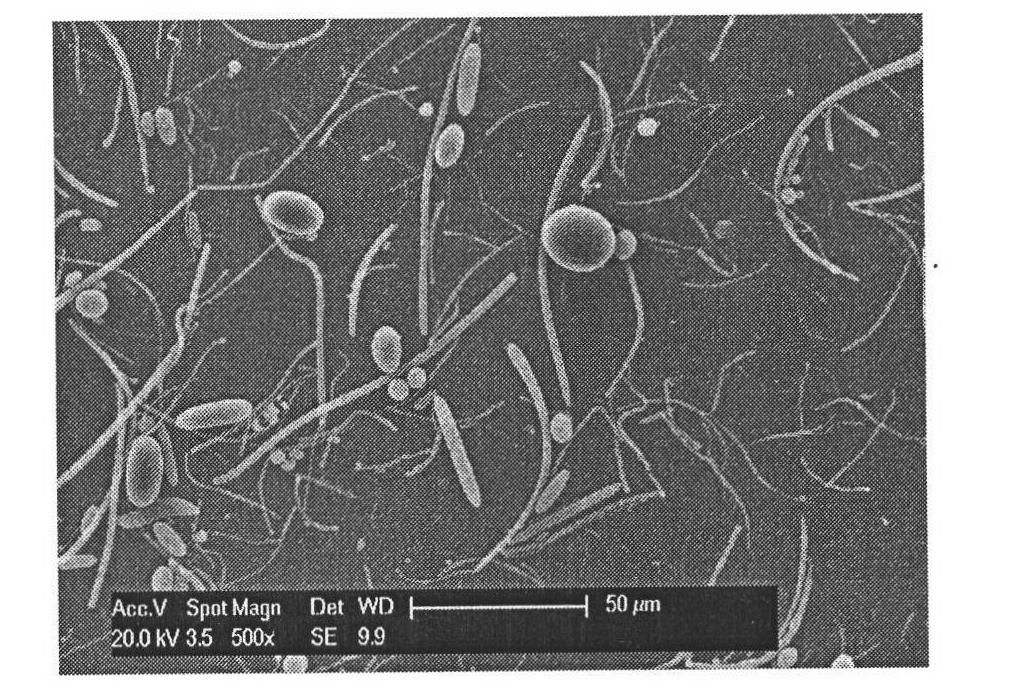
[0035] Weigh 100 mg of PLGA (molecular weight 5000) and 20 mg of all-trans retinoic acid (ATRA) into 10 mL of dichloromethane, stir at room temperature until dissolved; add 1.84 g of sodium polyphosphate (STP) to 0.5% PVA (Polymerization degree 550-650, alcoholysis degree 88%) Stir in the solution until dissolved. Mix 10 mL of dichloromethane solution containing ATRA and PLGA with 100 mL of PVA solution containing STP, stir at 800 rpm for 2 hours, centrifuge at 10,000 rpm, wash the surfactant with deionized water, and freeze-dry at -30°C to obtain a light yellow powder. The shape of drug-loaded particles is fibrous, see attached image 3 . Such as Figure 4 Curve d shows a slow release over a period of 4 weeks with a cumulative release rate of 7.5%. Compared with the ATRA microspheres prepared without adding small molecule excipients under the same conditions, the drug loading and encapsulation efficiency increased from 8.83%, 48.4% to 17.9% and 89.9%, respectively. Figur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com