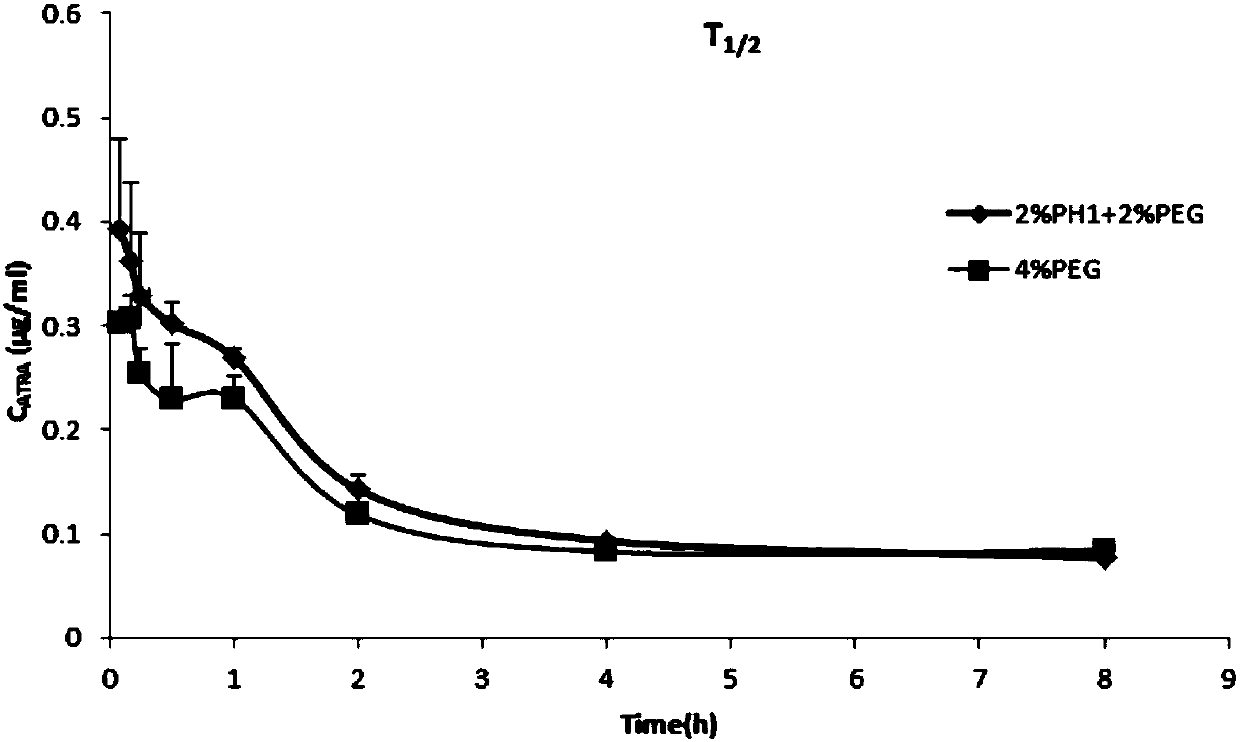
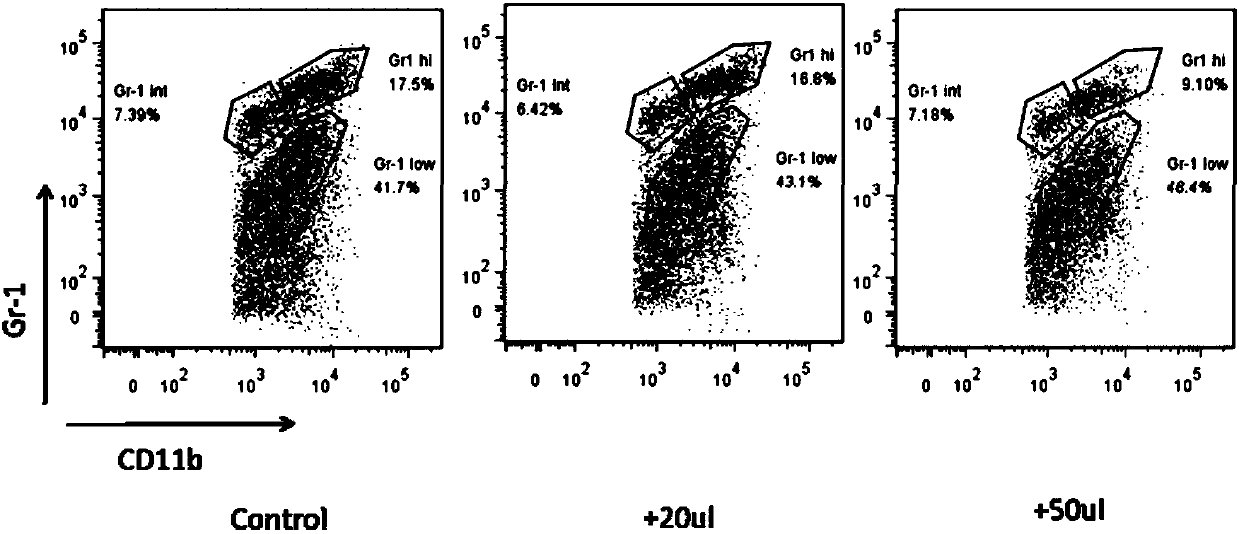
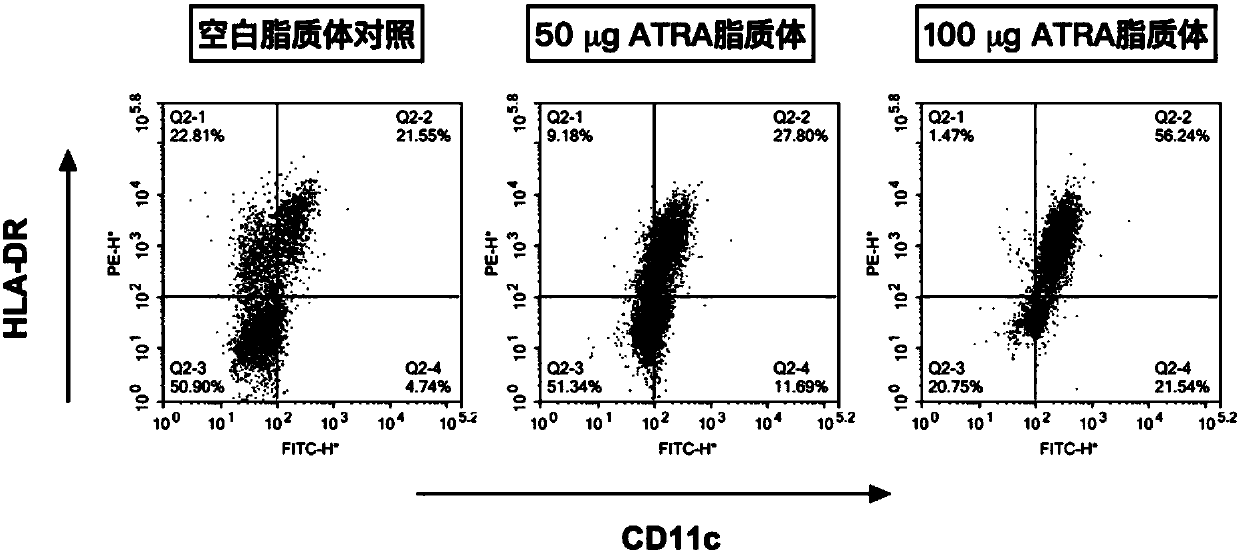
All-trans retinoic acid injection and application
一种全反式维甲酸、注射剂的技术,应用在生物制药领域,能够解决全反式维甲酸血浆半衰期短、全反式维甲酸水溶性低、生物利用度只有30%等问题,达到抑制肿瘤细胞增值、大医疗实用性、抑制肿瘤复发的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] In this example, the following three methods are used to investigate the saturation solubility of different solubilizing molecules after solubilizing ATRA.
[0074] (1) Emulsification method: Dissolve an appropriate amount of solubilizing molecules in 1ml ultrapure water as the water phase; dissolve 1mg of the drug in 1ml of an organic solvent as the organic phase; add the organic phase to the water phase under stirring, Stir overnight to evaporate the organic solvent or remove the organic solvent by rotary evaporation to obtain a drug-containing solution.
[0075] (2) Dialysis method: Dissolve the drug and solubilizing molecules in an organic solvent, then mix with 1 ml of ultrapure water, and dialyze the obtained solution in pure water to obtain a solution containing the drug.
[0076] (3) Freeze-drying method: dissolve an appropriate amount of solubilizing molecules in 1ml of pure water, add an appropriate amount of freeze-drying protective agent, as the water phase;...
Embodiment 2
[0080] EPC, HSPC and DPPC were selected as the main lipid materials respectively. Mix according to the molar ratio of PC:Chol:DSPE-PEG2000=2:1:0.125, add ATRA (the mass ratio of lipid / ATRA is 20:1, 40:1, 50:1 respectively), and add 5-7 small glass beads and hydrated by swirling for 30 minutes. Sequentially extrude through polycarbonate membranes with apertures of 400nm, 200nm, and 100nm 15 times each. The obtained all-trans retinoic acid liposomes had an average particle size in the range of 100nm±30nm and a PDI of about 0.1. Remove free all-trans retinoic acid (ATRA) by Sephadex G-50 microcolumn, within the linear range of the standard curve of all-trans retinoic acid, by removing the UV absorption peak area of ATRA encapsulated into liposomes The encapsulation efficiency of the ATRA liposome obtained by the ratio of the UV absorption peak area of ATRA before removal is respectively EPC liposome: 94%; HSPC liposome: 91%; DPPC liposome: 76%. The concentration of ATRA in...
Embodiment 3
[0083] Weigh 0.097 gram of hydrogenated soybean lecithin (HSPC), 0.031 gram of pegylated phospholipid 1,2-distearoylphosphatidylethanolamine-polyethylene glycol 2000 (DSPE-PEG2000) and 0.031 gram of cholesterol, and use 1.6 ml of Dissolve ethanol and place in a 70°C water bath to dissolve and mix. The ethanol mixture was added to 6.4 mL of calcium acetate buffer (pH 9.0). And placed in a water bath at 70 degrees Celsius for 30 minutes. The obtained liposome vesicles are sequentially extruded through polycarbonate membranes with apertures of 400nm, 200nm, 100nm, and 50nm each eight times to finally obtain liposomes with an average particle diameter of about 90nm.
[0084] Dialyze the liposomes prepared in the previous step through a dialysis membrane with a pore size of 10,000, replace the aqueous phase with a 10% mass fraction, and add a suspension of 4 mg / ml all-trans retinoic acid to a sucrose solution with a pH of 6 to 7 , and incubated at 60 degrees Celsius for 45 minute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com