Combination Therapies with Anti-CD38 Antibodies
an anti-cd38 and anti-cd38 technology, applied in the direction of antibody medical ingredients, drug compositions, peptides, etc., can solve the problems of no evidence of a cure, only a small overall survival, and multiple tumors and lesions throughout the skeletal system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Methods
Antibodies and Reagents
[0192]A human mAb against an innocuous antigen (HIV-1 gp120) was used as an isotype control as described previously (van der Veers et al., Haematologica 96:284-290, 2011; van der Veers et al., Blood Cancer J 1:e41, 2011). All-trans retinoic acid (ATRA) was purchased from Sigma-Aldrich and diluted in DMSO.
Bioluminescence Imaging (BLI)-Based ADCC Assays Using Luciferase (LUC)-Transduced MM Cell Lines
[0193]LUC-transduced MM cell lines were co-cultured with effector cells (freshly isolated PBMCs from healthy donors) at an effector to target ratio of 1:25 in white opaque 96-well flat bottom plates (Costar) in the presence of daratumumab (0.001, 0.01, 0.1, and 1.0 μg / mL) for four hours. The survival of LUC+-MM cells was then determined by BLI, 10 minutes after addition of the substrate luciferin (125 μg / mL; Promega). Lysis of MM cells was determined using the following formula: % lysis=1−(mean BLI signal in the presence of effector cells and daratumum...
example 2
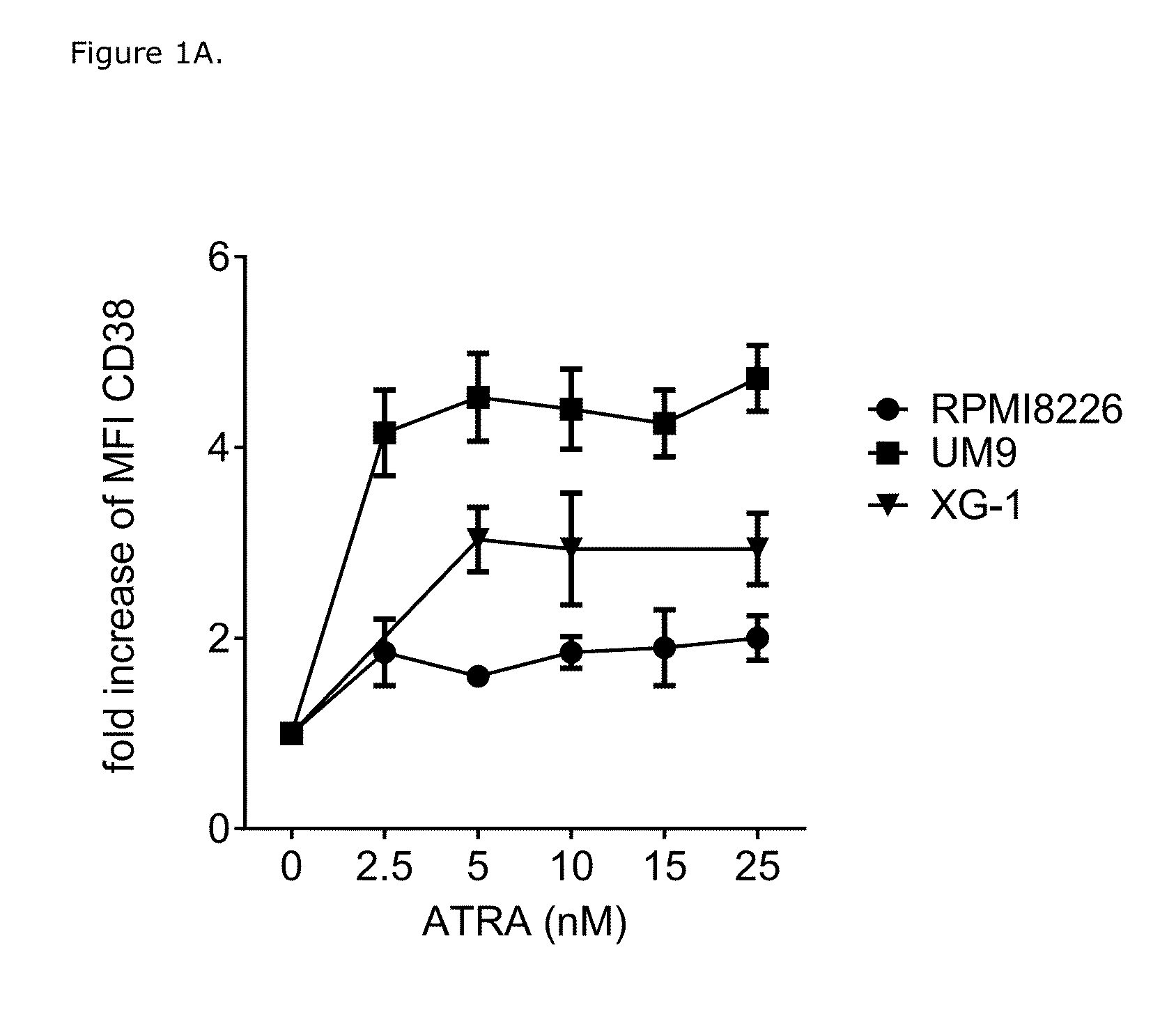
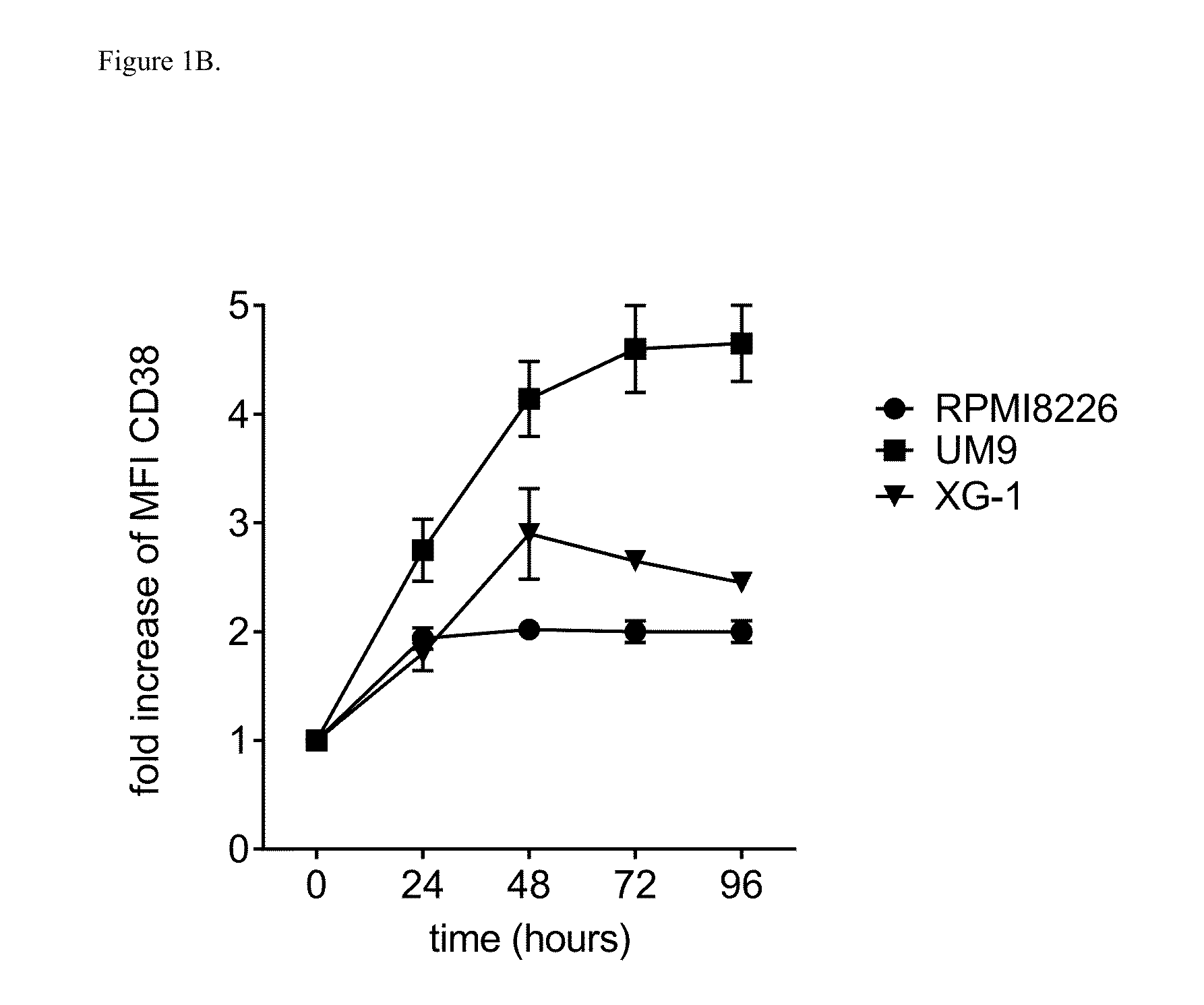
ATRA Increases CD38 Expression on MM Cell Lines and in Primary MM Cells
[0198]An increase in CD38 expression levels may enhance the efficacy of daratumumab to kill MM cells via ADCC or CDC. Interaction of ATRA with nuclear retinoic acid receptors results in altered expression of target genes including induction of CD38 expression (Malavasi F. J Leukoc Biol 90:217-219, 2011; Drach et al., Cancer Res 54:1746-1752, 1994). Therefore, effect of ATRA on MM cell lines RPMI8226, UM9, and XG1 was studied. MM cells were incubated with RPMI-1640 medium alone or with ATRA ranging from 0-25 nM for 48 hours (FIG. 1A) or were incubated with 10 nM ATRA for 24, 48, 72 or 96 hours (FIG. 1B) and then harvested to determine CD38 expression by flow cytometry using a FACS-Calibur device (Becton Dickinson) and anti-CD38 antibody (Beckman Coulter). The data were analyzed using the CellQuest software.
[0199]Minimum of 10 nM ATRA was sufficient to induce a 1.9-4.4-fold increase in CD38 expression on the MM cel...
example 3
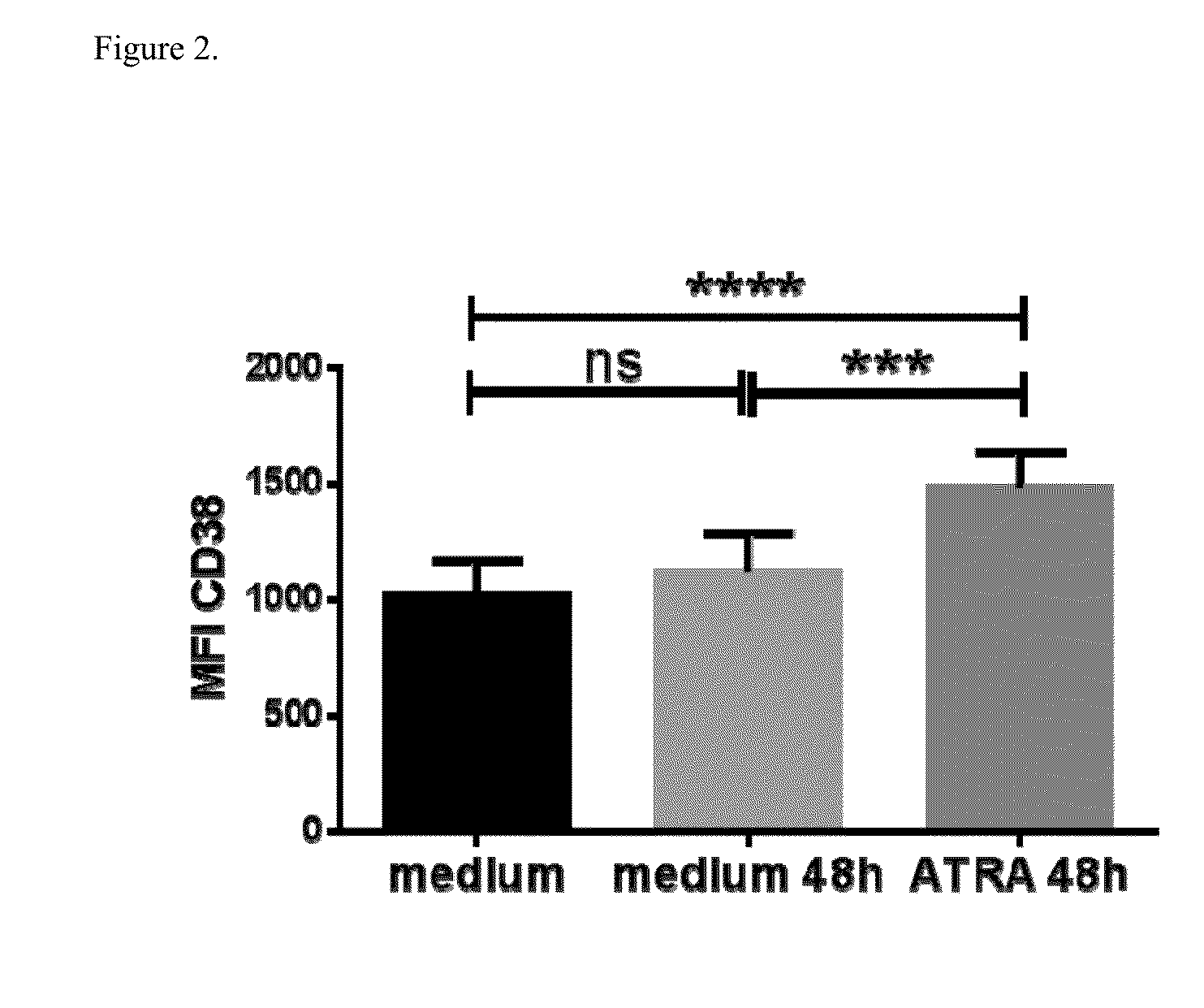
ATRA-Mediated Upregulation of CD38 Enhances Both Daratumumab-Mediated ADCC and CDC Against MM Cells
[0202]Possible effect of ATRA-induced upregulation of CD38 expression on daratumumab-induced ADCC and CDC was tested in MM cell lines XG-1, RPMI8226 and UM9 and in primary MM cells.
[0203]For MM cell lines, CDC and ADCC were assessed using bioluminescence imaging (BLI) based ADCC and CDC assays as described above. For primary MM cells, CDC and ADCC were assessed using Flow cytometry-based ex vivo ADCC and CDC assays in BM-MNC as described above. In the assays, cells were pre-treated with 10 nM ATRA or solvent control for 48 hours, followed by incubation with or without daratumumab in the presence of PBMCs as effector cells for assessment of ADCC or in the presence of human serum as complement source for analysis of CDC. Isotype control was added at 10 μg / ml, and 10% heat-inactivated serum was used as control for CDC.
[0204]FIG. 3A, FIG. 3B and FIG. 3C show the results of daratumumab-indu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com