Preparation method of deuterated benzene compound
A compound, deuterated benzene technology, applied in the field of deuterated compound synthesis, can solve problems such as substrate versatility limitation, and achieve the effects of simple and efficient synthesis method, simple and convenient operation process, and easy to obtain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The generation of embodiment 1 deuterated toluene
[0027]
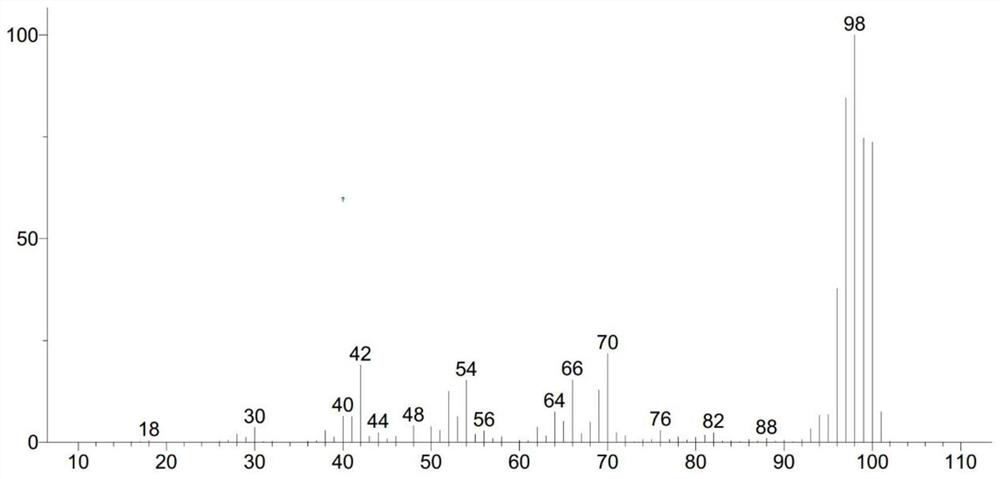
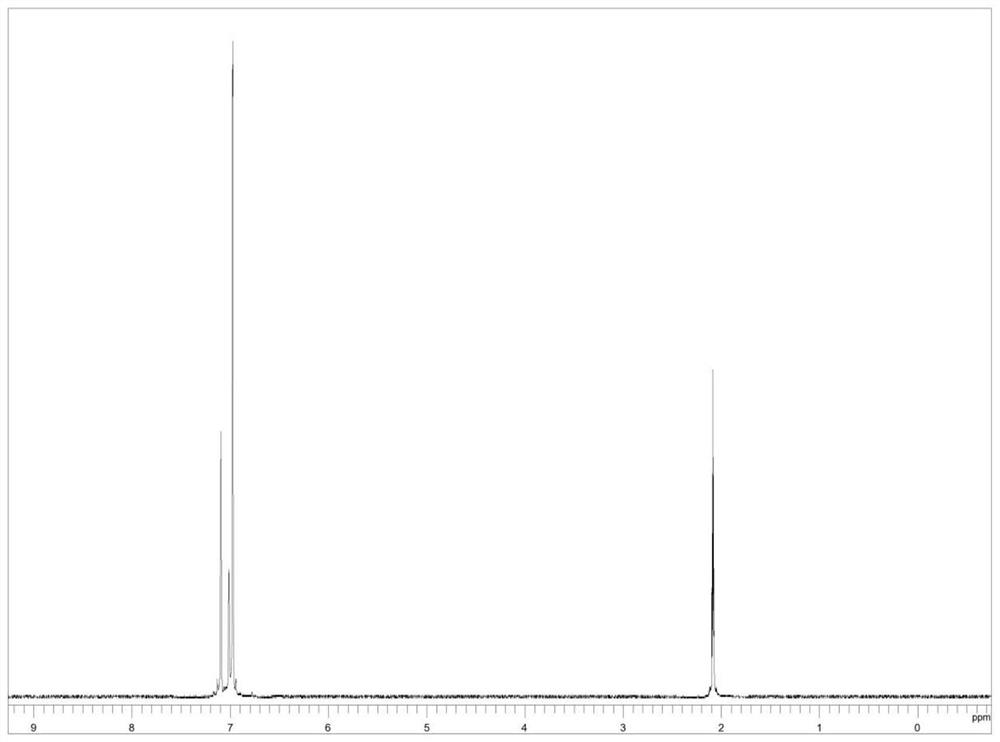
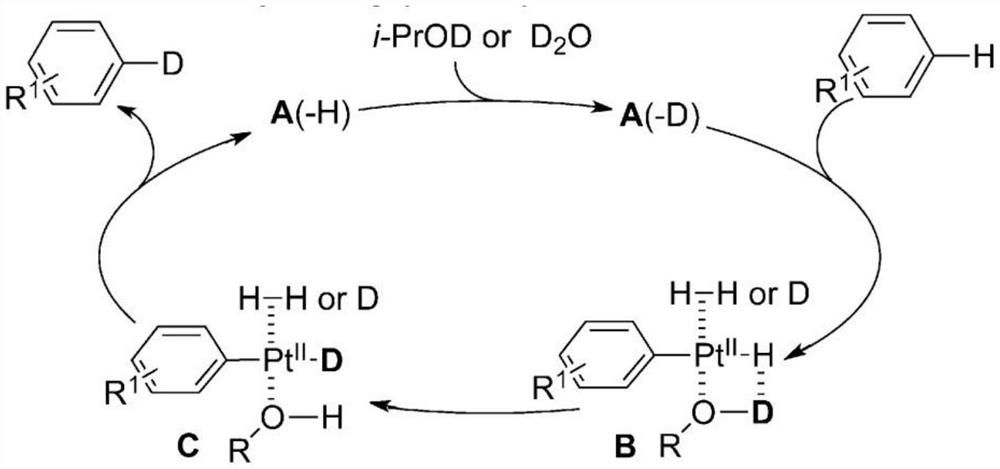
[0028] Add 0.25mmol (23.035mg) of toluene, 2mL of deuterium water, 24.38mg of 10% platinum carbon, 0.1mL of isopropanol and 0.9mL of cyclohexane solution into a 50mL three-necked flask equipped with a condenser, a thermometer and electromagnetic stirring, and use nitrogen gas Replace the air in the reaction system, then raise the temperature to 70°C, react at this temperature for 12 hours, then lower to room temperature, remove the catalyst platinum carbon by filtration, and obtain a mixture of deuterium water and crude product. 10 mL of dichloromethane was added to the mixture, the organic layer and the deuterium water layer were separated, the lower organic layer liquid was collected, and the organic layer liquid was rotary evaporated at 38° C. to obtain 17.184 mg of the product deuterated toluene liquid, with a yield of 74.6%. The MS spectrum of deuterated toluene is as follows figure 1 As shown, the NM...
Embodiment 2
[0030] Add 0.25mmol (23.035mg) of toluene, 2mL of deuterium water, 24.38mg of 10% palladium carbon, and 1mL of isopropanol solution into a 50mL three-necked flask equipped with a condenser, a thermometer and electromagnetic stirring, and replace the air in the reaction system with nitrogen , and then heated up to 70 ° C, reacted at this temperature for 12 hours and then lowered to room temperature, and the catalyst palladium carbon was removed by filtration to obtain a mixture of deuterium water and crude product. 10 mL of dichloromethane was added to the mixture, the organic layer and the deuterium water layer were separated, the lower organic layer liquid was collected, and the organic layer liquid was rotary evaporated at 38° C. to obtain 16.17 mg of the product deuterated toluene liquid, with a yield of 70.2%.
Embodiment 3
[0032] Add 0.25mmol (23.035mg) of toluene, 2mL of deuterium water, 24.38mg of 10% platinum carbon and 1mL of isopropanol in a 50mL three-necked flask equipped with a condenser tube, a thermometer and electromagnetic stirring, and replace the air in the reaction system with nitrogen, Then the temperature was raised to 70° C., reacted at this temperature for 12 hours, and then cooled to room temperature, and the catalyst platinum carbon was removed by filtration to obtain a mixture of deuterium water and crude product. 10 mL of dichloromethane was added to the mixture, the organic layer and the deuterium water layer were separated, the lower organic layer liquid was collected, and the organic layer liquid was rotary evaporated at 38° C. to obtain 16.1245 mg of the product deuterated toluene liquid, with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com