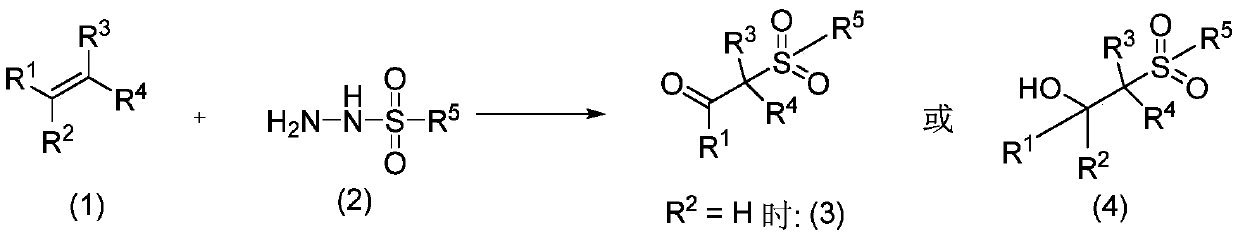
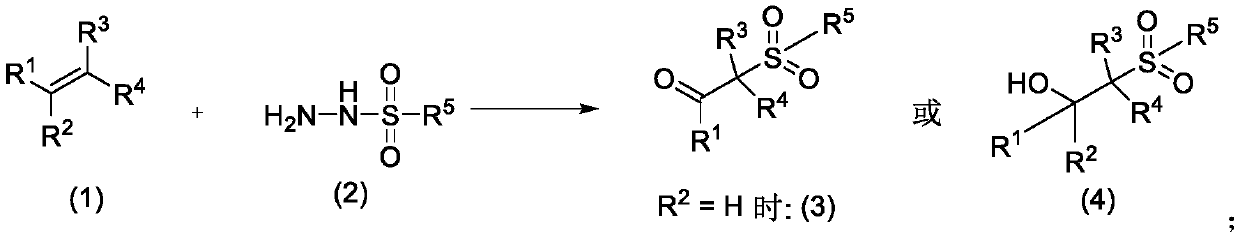
Method for preparing β-ketosulfone or β-hydroxysulfone by reaction of substituted alkenes and sulfonyl hydrazide derivatives
A technology of sulfonyl hydrazide and derivatives, applied in the field of organic chemical synthesis, to achieve the effects of easy preparation, simple process and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 2ml of solvent ethanol, 0.093g (0.5mmol) of p-toluenesulfonyl hydrazide, 0.156g (1.5mmol) of styrene, 0.112g (1mmol) of base triethylenediamine, and 3.2mg of photocatalyst methylene blue in a 10ml reaction tube. (0.01mmol), irradiated with 12W blue LED, exposed to normal temperature reaction. Follow the reaction until the complete disappearance of p-toluenesulfonyl hydrazide. After the reaction, it was extracted three times with water and ethyl acetate. The aqueous layer was removed, and the organic layer was dried over anhydrous sodium sulfate. The solvent was removed by a rotary evaporator, and then purified by silica gel chromatography (ethyl acetate / petroleum ether=1 / 10) to obtain 0.106 g of pure 2-p-toluenesulfonylacetophenone with a yield of 78%. 1 H NMR (400MHz, CDCl 3 )δ7.97(d, J=7.7Hz, 2H), 7.78(d, J=8.1Hz, 2H), 7.64(t, J=7.3Hz, 1H), 7.50(t, J=7.6Hz, 2H) ,7.35(d,J=8.0Hz,2H),4.74(s,2H),2.46(s,3H). 13 C NMR (101MHz, CDCl 3 ) δ 188.15, 145.36, 135.82, 134....
Embodiment 2
[0020] In Reaction Example 1, the reaction was carried out in the same manner as in Example 1, except that 0.156 g (1.5 mmol) of styrene was changed to 0.275 g (1.5 mmol) of 1-bromo-4-vinylbenzene. 2-p-toluenesulfonyl-4'-bromoacetophenone, yield 89%. 1 HNMR (400MHz, CDCl 3 )δ8.02(t, J=1.8Hz, 1H), 7.91(ddd, J=7.8, 1.6, 1.0Hz, 1H), 7.80–7.71(m, 3H), 7.38(dd, J=15.3, 7.8Hz ,3H),4.70(s,2H),2.47(s,3H). 13 C NMR (101MHz, CDCl 3 ) δ 186.69, 144.92, 137.41, 132.04, 130.34, 128.55, 127.98, 123.18, 63.98.
Embodiment 3
[0022] In Reaction Example 1, the reaction was carried out in the same manner as in Example 1, except that 0.156 g (1.5 mmol) of styrene was changed to 0.275 g (1.5 mmol) of 1-bromo-3-vinylbenzene. 2-p-toluenesulfonyl-3'-bromoacetophenone, yield 67%. 1 HNMR (400MHz, CDCl 3 )δ8.02(t, J=1.6Hz, 1H), 7.91(d, J=7.8Hz, 1H), 7.76(t, J=6.9Hz, 3H), 7.38(dd, J=15.3, 7.8Hz, 3H), 4.70(s,2H), 2.47(s,3H). 13 C NMR (101MHz, CDCl 3 )δ 186.98, 145.64, 137.41, 137.12, 135.54, 132.09, 130.40, 129.95, 128.60, 128.03, 123.18, 63.68, 21.75.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com