O-aminoalcohol compounds, preparation method thereof and application thereof
An o-amino alcohol and compound technology, which is applied in the field of o-amino alcohol compounds and their preparation, can solve the problems of low content and affect inflammation resistance, and achieve the effects of simple operation and separation, high efficiency and selectivity, and cheap and easy-to-obtain raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] 1. Preparation of FAAH enzyme buffer:
[0037] Tris buffer (50 mM, pH 8.0) / fatty acid-free bovine serum albumin (BSA, 0.05%, as prepared).
[0038] 2. Prepare the recombinant protein of FAAH enzyme:
[0039] 1) Preparation method: The gene of FAAH enzyme was transferred into HEK-293 cells by genetic engineering method, the expressed cells were screened with G418, and then the corresponding protein was extracted from the cells after cell culture.
[0040] 2) Extraction method: cells were washed twice with PBS, 20 mM tris-HCL (pH 7.5, containing 0.32 M glucose) buffer solution was added and sonicated; the supernatant was collected by centrifugation at 800 g for 15 min. The protein concentration was measured by BCA method, diluted to 1 mg / ml, and frozen at -80 for later use.
[0041] 3. The specific steps of the activity experiment are:
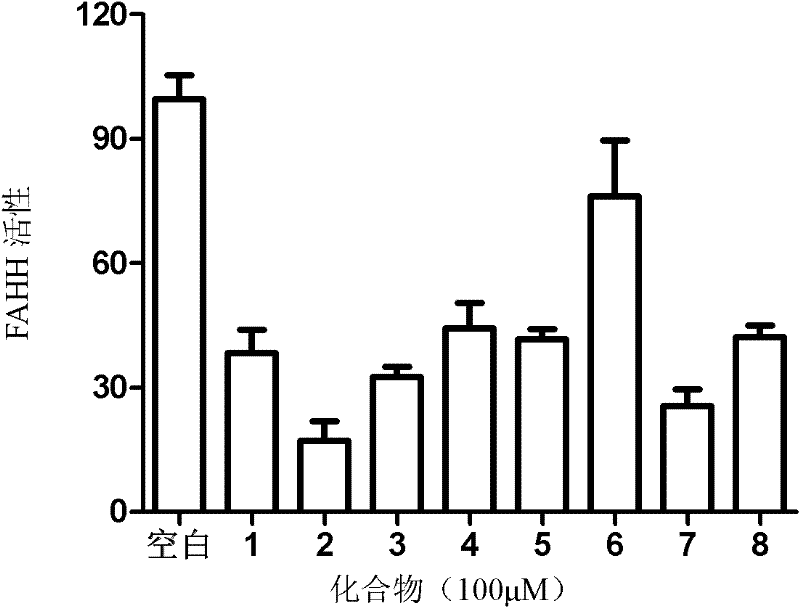
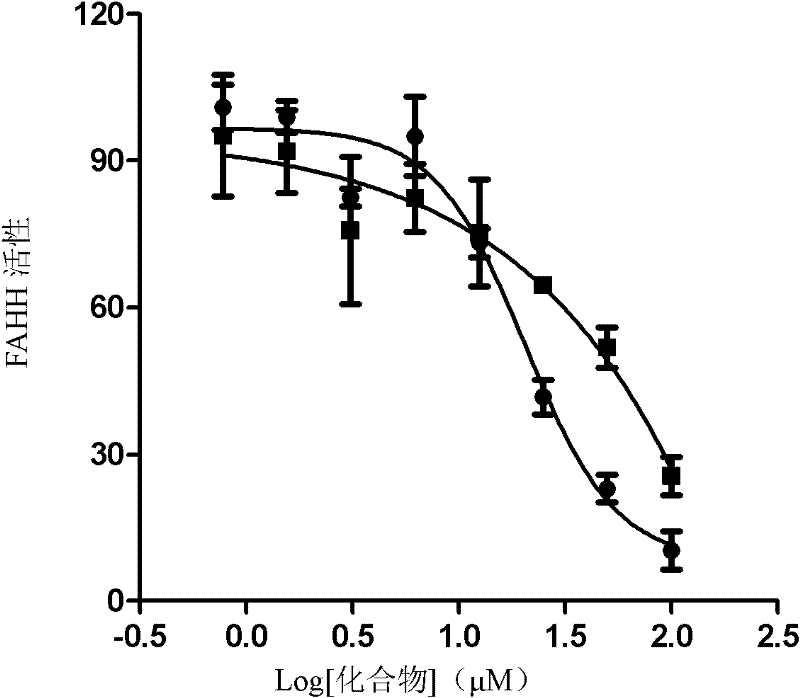
[0042] 1) Add 50 μL (50 μg) of the prepared enzyme into the injection bottle, then add 2 μL DMSO or different drugs (100 μM) and reac...
Embodiment 1
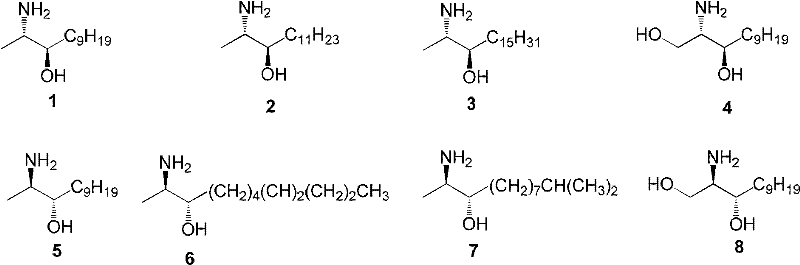
[0046] Example 1 Synthesis of (2S, 3R)-2-aminododec-3-ol (1)
[0047] Step 1. L-Alanine (750 mg, 8.4 mmol), K 2 CO 3 (4.05 g, 29.6 mmol) was dissolved in acetone (16.8 mL) solvent, BnCl (3.4 mL, 29.6 mmol) was added to the mixture under ice bath, heated, and the temperature was controlled at 50-70 °C and refluxed for 15 h. After the reaction was completed, the reaction mixture was cooled to room temperature, and the layers were separated. The upper organic phase was concentrated, and flash column chromatography (ethyl acetate: petroleum ether=1: 45) was used to obtain compound 10a (2.7 g, 7.5 mmol) in 89% yield. . [α] D 20 -88.2(c 1.0, CHCl 3 );IR(film)v max : 3061, 3024, 2936, 2835, 1729, 1494, 1448, 1375, 1189, 1140cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ: 1.32(d, J=7.1Hz, 3H), 3.54(q, J=7.1Hz, 1H), 3.61(d, J=14.0Hz, 2H), 3.81(d, J=14.0Hz, 2H) , 5.13 (d, J=12.3Hz, 1H), 5.21 (d, J=12.3Hz, 1H), 7.23-7.41 (m, 15H); 13 C NMR (100MHz, CDCl 3 )δ: 14.9, 54.4, 56.2, 66.0, 126....
Embodiment 2
[0051] Example 2 Synthesis of (2S, 3R)-2-aminotetradec-3-ol (2)
[0052] Except for step 3 and step 4, the remaining steps are the same as in Example 1.
[0053] In step 3, compound 12b was prepared from compound 11a using n-C 11 H 23 MgBr instead of n-C 9 H 19 MgBr. [α] D 20 +20.4(c 0.5, CHCl 3 ).IR(film)υ max : 3361, 2922, 2847, 1602, 1453, 1366, 1084cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ: 0.92(t, J=6.9Hz, 3H), 1.13(d, J=6.8Hz, 3H), 1.18-1.32(m, 19H), 1.68-1.78(m, 1H), 1.79(s, 1H) ), 2.71-2.77(m, 1H), 3.51(d, J=13.8Hz, 2H), 3.59-3.67(m, 1H), 3.80(d, J=13.8Hz, 2H), 7.19-7.41(m, 10H); 13 C NMR (100MHz, CDCl 3 )δ: 8.7, 14.1, 22.7, 25.9, 29.4, 29.6, 29.6, 29.6, 29.6, 29.7, 31.9, 34.3, 54.8, 57.3, 73.7, 126.9, 128.2, 128.8, 140.2ppm; MS(ESI) m / z 410 (M+H + , 100).Anal.calcd.for C 28 H 43 NO: C, 82.09; H, 10.58; N, 3.42. Found: C, 82.45; H, 10.33; N, 3.64.
[0054] In step 4, compound 2 was prepared by substituting compound 12b for 12a. [α] D 20 +12.0(c 0.5, MeOH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com