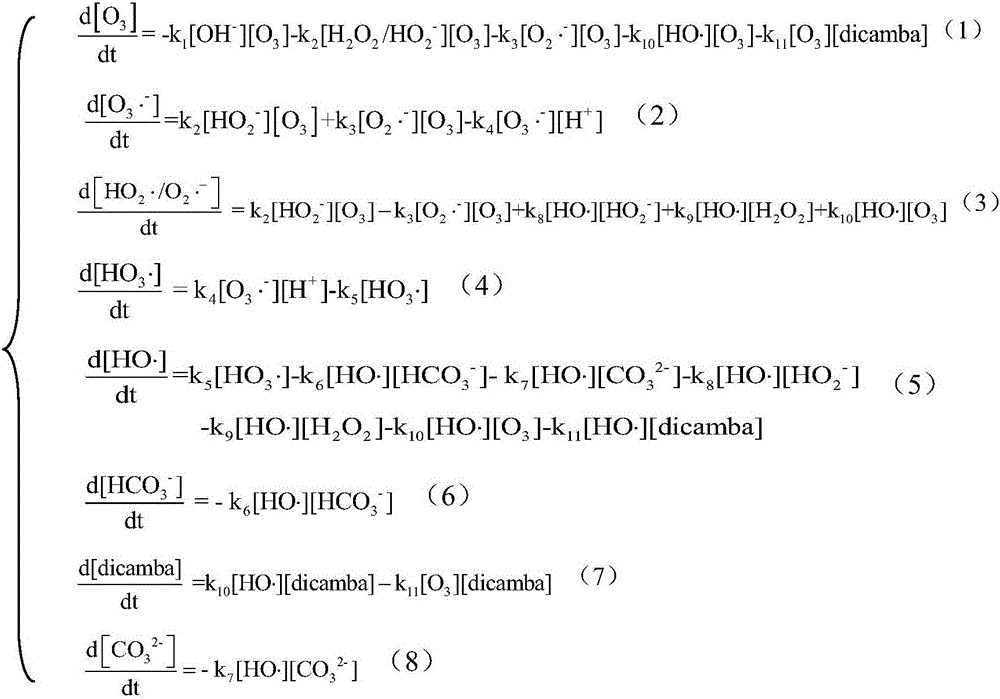Method for predicting oxidative degradation for removing dicamba in organic wastewaterofozone/hydrogen peroxide
A technology for oxidative degradation of organic wastewater, applied in chemical instruments and methods, water/sewage treatment, oxidized water/sewage treatment, etc., can solve the problem of the inability to establish a kinetic model of dicamba organic wastewater oxidative degradation and the inability to detect free radical degradation intermediate products etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] a) The reaction of ozone / hydrogen peroxide and dicamba starts with the chain reaction of producing hydroxyl radicals, including:
[0023] (1) Chain initiation reaction: ozone and OH in water - and the HO provided by hydrogen peroxide 2 - The product is O 3 - ·;
[0024]
[0025] (2) chain transfer reaction:
[0026] o 3 - After a series of chain transfer reactions, hydroxyl radicals are finally generated;
[0027]
[0028]
[0029]
[0030] (3) Chain termination reaction:
[0031] The generated hydroxyl radicals react with themselves, ozone and ions in solution;
[0032]
[0033]
[0034]
[0035]
[0036]
[0037] b) Reaction of dicamba with free radicals:
[0038] The reaction process of dicamba and free radicals produces intermediate products that eventually mineralize into carbon dioxide and water
[0039] HO·+Dicamba→Intermediate 1→Intermediate 2→…Intermediate n→CO 2 +H 2 o
[0040] c) Reaction of dicamba with ozone:
[004...
Embodiment 2
[0079] 1. 0.65mol / L ozone saturated solution configuration:
[0080] Put the aerator head of Akcome Y-45 ozone generator into 100ml deionized water for 30 minutes. Obtain 0.65mol / L ozone saturated solution, quickly transfer to a 50ml volumetric flask, and refrigerate at 5°C. Dilute with deionized water to obtain 0.35mM, 0.50mM, 0.065mM ozone solutions respectively.
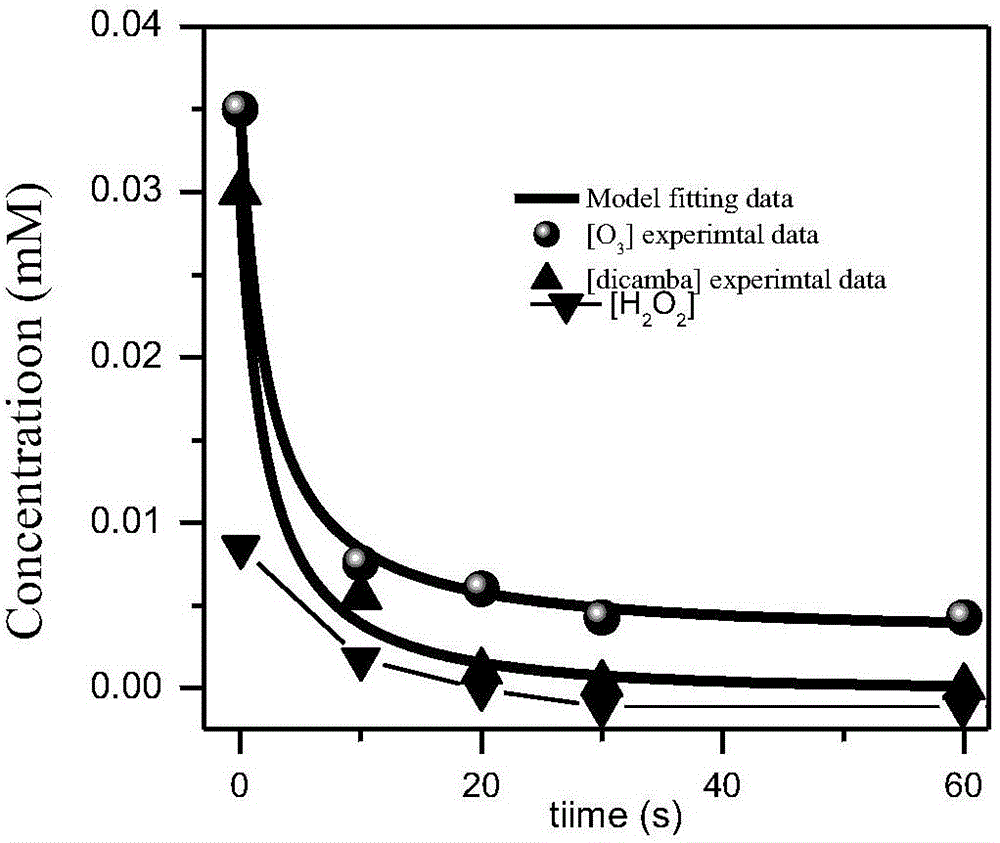
[0081] 2. Take 1ml of ozone solution (three concentrations, three times respectively) and 1ml of 30% hydrogen peroxide solution in volume concentration and add it to 100ml of dicamba simulated wastewater prepared with deionized water (dosing amount of dicamba is 0.0221g). Sampling and detection by the method of Example 1; finally, the sample is subjected to quantitative analysis.
[0082] The experimental observation value is: hydrogen peroxide concentration, ozone concentration and hydroxyl radical concentration, the correlation coefficient of experimental value and theoretical value (seeing table 2) is all gre...
Embodiment 3
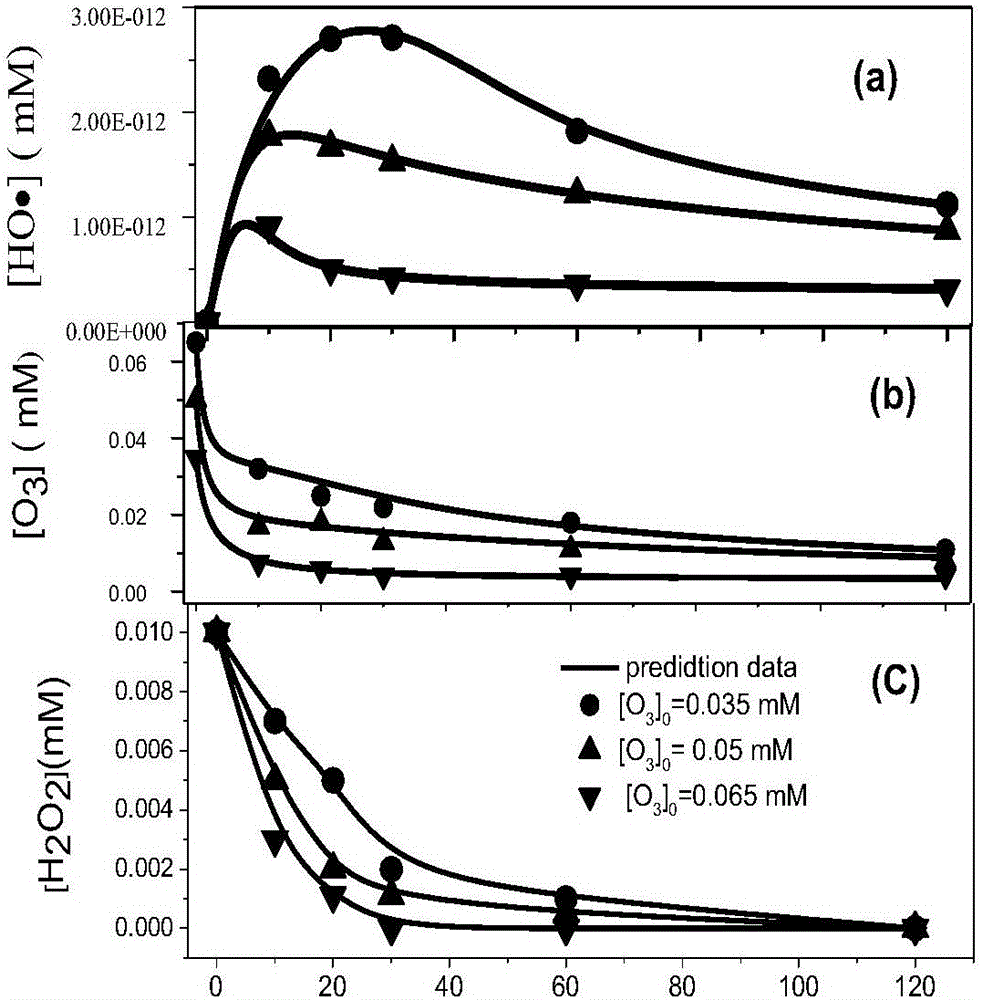
[0095] The deionized water in Example 2 was changed to river water, and the river water was collected according to the national standard: [JGJ63-2006]. The on-site measurement pH was 6.9. The Qiantang River water was sampled at a depth of 3m in the center of the river, and the shelf life was 7 days. Water quality: chloride ion: 2mg / L, iron ion: 0.05mg / L, carbonate: 40mg / L, DOC: 0.7mg / L. Other operations are the same as in Example 2, with natural water as the background, as a verification of the model, as a result, dicamba concentration, hydrogen peroxide concentration, ozone concentration and hydroxyl radical concentration, the correlation coefficients of experimental values and theoretical values are all greater than 0.95, ozone, hydrogen peroxide, The experimental value of hydroxyl radical concentration is close to the theoretical value, and the established kinetic model is reliable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com