A kind of preparation method of resveratrol compound
A technology of resveratrol and compounds, which is applied in the field of preparation of resveratrol compounds, can solve the problems of poor selectivity of resveratrol ethers, difficult configuration of carbon-carbon double bonds, and strong irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a kind of preparation method of resveratrol compound, comprises the steps:
[0027] (1) Alkoxy substituted benzyl halides, alkoxy substituted benzaldehydes and metal catalysts undergo oxidative addition and reduction elimination reactions to obtain alkoxy substituted benzophenones;
[0028] (2) Reduction, trans-elimination and selective debenzylation of the alkoxy-substituted benzophenone and a metal catalyst occur in a hydrogen atmosphere to obtain resveratrol compounds.
[0029] The reaction formula of the preparation method provided by the present invention is shown in formula (1):
[0030]
[0031] Wherein, X is Cl, Br or I, R 1 and R 3 independently methoxy or benzyloxy, R 2 and R 4 independently methoxy, benzyloxy or hydrogen, R 5 and R 7 independently methoxy or hydroxy, R 6 and R 8 are independently methoxy, hydroxy or hydrogen. In the preparation method of the present invention, hydrogenation reduction, trans elimination and s...
Embodiment 1
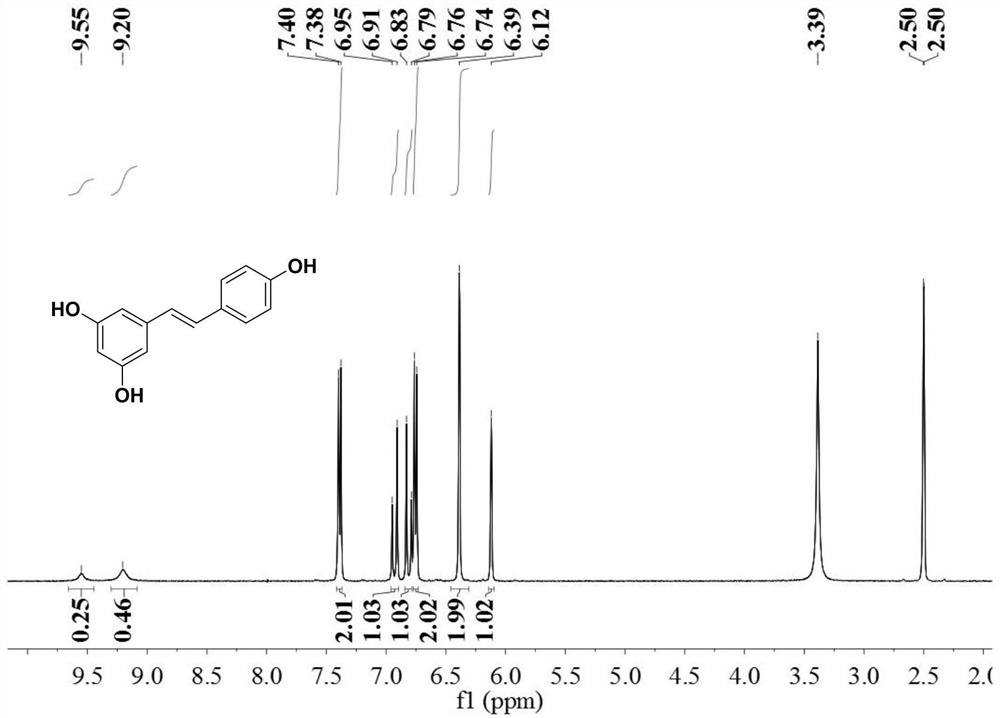
[0056] 67.7 grams of 3,5-dibenzyloxybenzyl chloride, 42.4 grams of 4-benzyloxybenzaldehyde, 2 grams of 5wt% palladium carbon catalyst and 400 grams of isopropanol were mixed, heated to 80 ° C for 4 hours, and the The resulting reaction solution was cooled to below 25°C; the gas in the reaction vessel was replaced with hydrogen, and then hydrogen was introduced to a pressure of 8kg f / cm 2 Then, the temperature was raised to 100°C, and the reaction was continued for 5 hours; the resulting reaction solution was filtered to recover the catalyst, the filtrate was evaporated to dryness, and the evaporated solid was heated to 80°C with 50 grams of toluene to dissolve, then cooled to 10°C, filtered and dried to obtain 40.5 gram of resveratrol, the yield is 88.8%, and the purity tested by liquid chromatography is 99.6%.
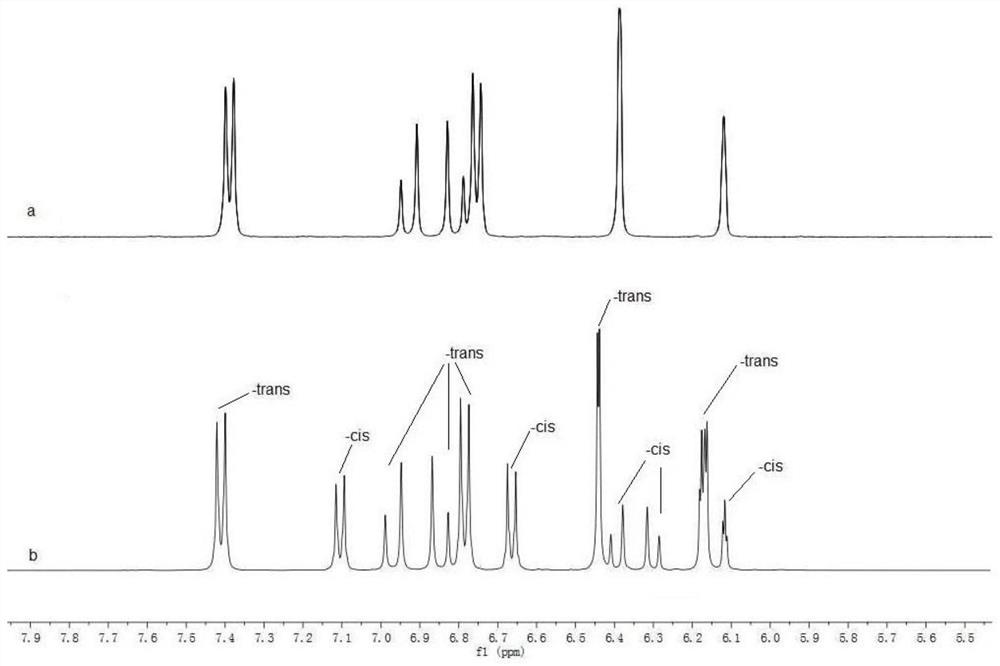
[0057] The product obtained in this embodiment is characterized by NMR, and its hydrogen spectrogram is as follows: figure 1 As shown, compare it with figure 2 Com...
Embodiment 2
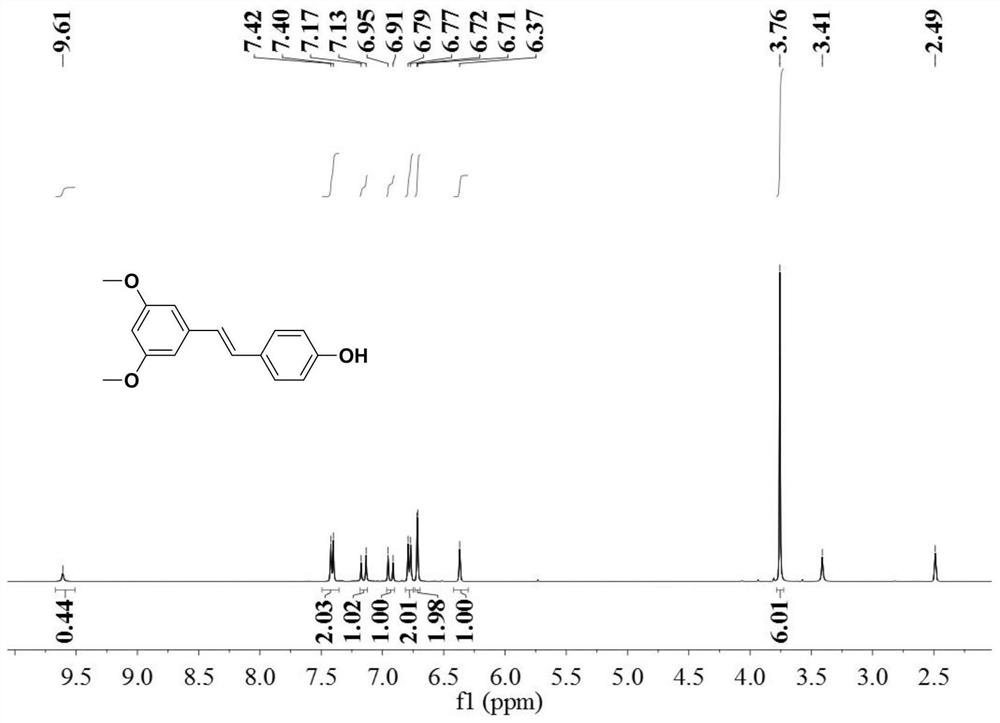
[0059] 37.3 grams of 3,5-dimethoxybenzyl chloride, 42.4 grams of 4-benzyloxybenzaldehyde, 4.24 grams of 5wt% palladium carbon catalyst and 400 grams of toluene were mixed, heated to 120 ° C for 3 hours, and the resulting reaction Cool the solution to below 25°C; replace the gas in the reaction vessel with hydrogen and feed hydrogen to a pressure of 8kg f / cm 2 , then heated up to 150°C, and continued to react for 3 hours; the obtained reaction solution was filtered to recover the catalyst, the filtrate was evaporated to dryness, and the evaporated solid was heated to 85°C with 50 grams of toluene to dissolve, then cooled to 5°C, filtered and dried to obtain 44.5g Pterostilbene, the yield is 86.9%, and the purity is 99.5% by liquid chromatography.
[0060] The product obtained in this embodiment is characterized by NMR, and its hydrogen spectrogram is as follows: image 3 shown by image 3 It can be seen that the obtained pterostilbene is in the trans structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com