Adenaphtho-imidazolyl nitrogen heterocyclic carbene metal palladium complex catalyst and preparation and application thereof
A technology of acenaphthoimidazolyl nitrogen and heterocyclic carbene, which is applied in the field of metal ligand catalysts and chemical synthesis, to achieve the effects of mild reaction conditions, promotion of catalytic cycle, and enhancement of σ electron donating ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Synthesis of acenaphthoimidazolyl nitrogen heterocyclic carbene metal palladium complex Pd-NHC-1
[0040] (1) Synthesis of α-diimine compounds: Weigh 0.7g (3.84 mmol) of acenaphthenequinone into a 100mL three-neck flask, add 35mL of acetonitrile and heat at 80°C for 15 minutes, then add 6.5mL of glacial acetic acid. Also in a beaker, dissolve 1.63 g (9.2 mmol) of 2,6-diisopropylaniline with 30 mL of acetonitrile and pour it into a constant pressure burette. After the acenaphthylquinone is completely dissolved, slowly drop the acetonitrile solution substituted for aniline into the three-necked flask, the reaction device is carried out under the protection of nitrogen, continue to heat and react for 5 hours, cool at room temperature, filter, and wash with n-hexane to obtain α-diimine compounds The target product is 1.78 g, and the yield is 92%.
[0041](2) Synthesis of azacyclic carbene salt (imidazolium salt): add 1.00 g (2 mmol) of α-diimine compound, 3.2...
Embodiment 2
[0045] Embodiment 2: Synthesis of acenaphthoimidazolyl nitrogen heterocyclic carbene metal palladium complex Pd-NHC-2
[0046] 3-chloropyridine is replaced by pyridine, and others are the same as in Example 1. The acenaphthoimidazolyl nitrogen heterocyclic carbene metal palladium complex Pd-NHC-2 was obtained, yield: 0.280 g. Its NMR characterization data are as follows:
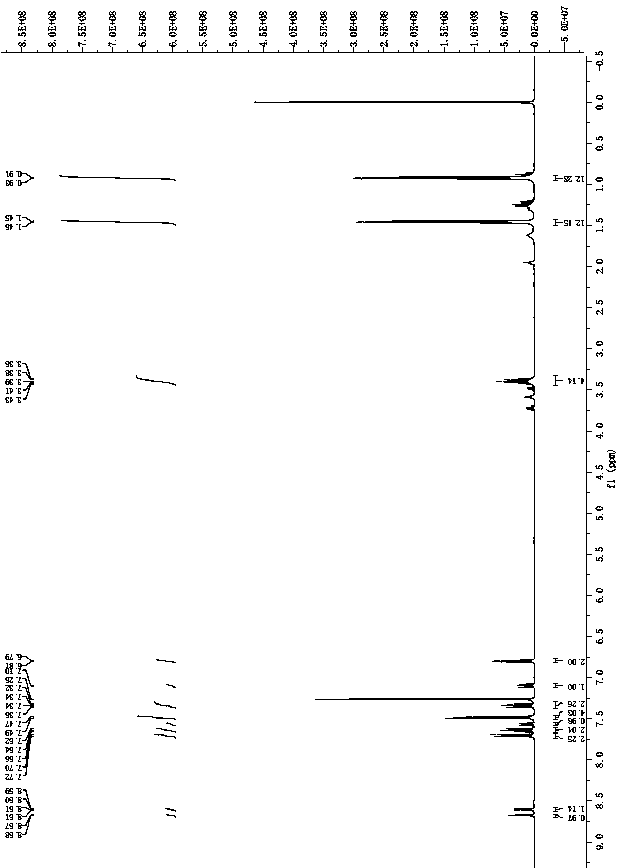
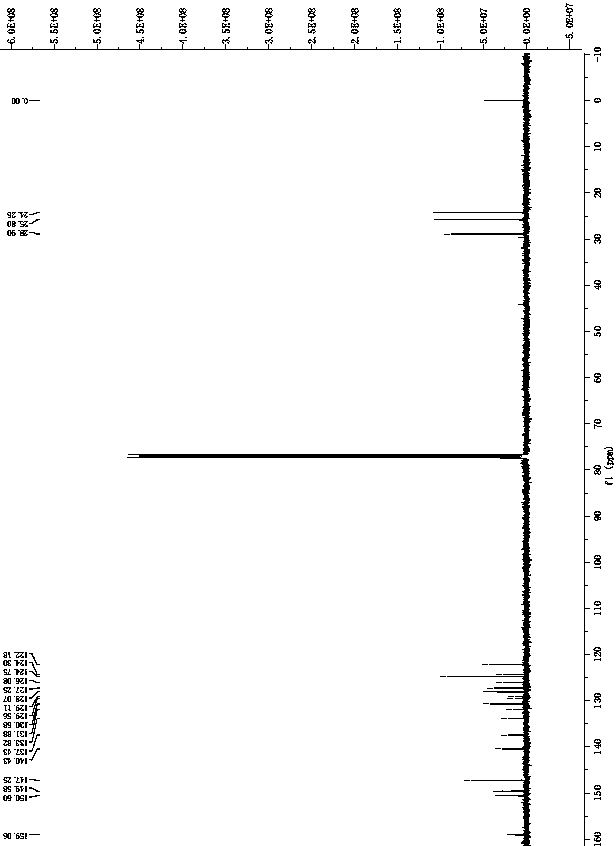
[0047] 1 H NMR (400 MHz, CDCl 3 ) δ 8.64 (d, J = 5.0 Hz, 2H), 7.70 (d, J = 8.3 Hz,2H), 7.63 (t, J = 7.8 Hz, 2H), 7.56 (s, 2H), 7.48 (d, J = 7.8 Hz, 4H), 7.34(m, 2H), 7.13 (m, 2H), 6.79 (d, J = 7.0 Hz, 2H), 3.42 (m, 4H), 1.46 (d, J =6.6 Hz, 12H), 0.92 (d, J = 6.9 Hz, 12H). 13 C NMR (101 MHz, CDCl 3 ) δ 151.58(s), 147.26(s), 130.59(s), 127.99(s), 127.24(s), 124.71(s), 123.97(s),122.12(s), 28.89(s), 25.79(s ), 24.29 (s).
Embodiment 3
[0048] Embodiment 3: Synthesis of 2-phenylquinolin-4(1H)-one
[0049] In an autoclave with a volume of 75 mL, add 5 mL of N,N-dimethylformamide, 1 mmol of o-iodoaniline, 1.2 mmol of phenylacetylene, 4.0 mmol of dimethylamine, and 0.5 mol% of acenaphthoimidazolyl nitrogen Heterocyclic carbene metal palladium complex Pd-NHC-1 (relative to ortho-iodoaniline). Seal the reactor, replace the reactor with carbon monoxide 3 times, and seal the reactor. The CO gas pressure is 2.0 MPa, and the temperature is controlled by the temperature controller to slowly rise to 100 o C, reacted for 15 hours, cooled to room temperature, unloaded, and the liquid obtained by the reaction was qualitatively analyzed with an Agilent 6890 / 5973 gas chromatography spectrometer, the selectivity of the target product 2-phenylquinolin-4(1H)-one was greater than 99%, separated The yield is 95%. Product characterization data are as follows:
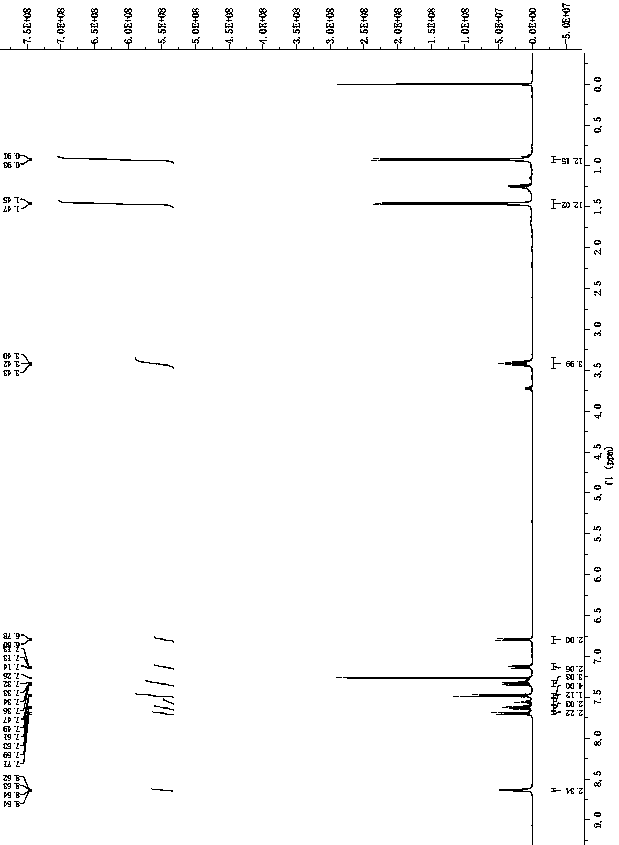
[0050] 1 H NMR (400 MHz, DMSO) δ 11.74 (s, 1H), 8.11 (m, 1H), 7.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com