Nano-composite material and preparation method thereof
A nanocomposite material, NH2-MIL-125 technology, applied in the field of preparation of materials for removing uranyl ions, achieving the effect of good yield and removal effect, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
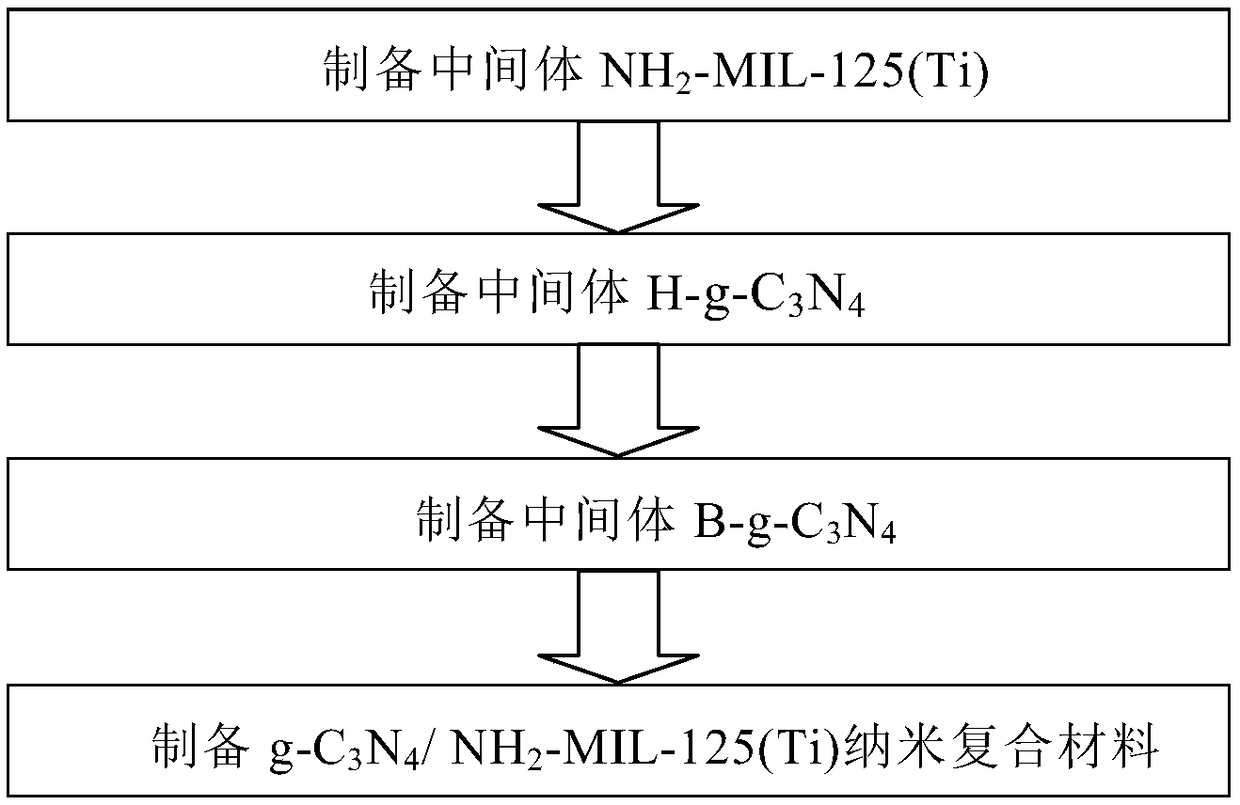
[0048] Such as figure 1 As shown in the flow chart of a preferred embodiment of the present invention, to obtain final product g-C 3 N 4 / NH 2 -MIL-125(Ti) nanocomposite material, the intermediate NH needs to be prepared first 2 -MIL-125(Ti), thermally oxidized graphene-like carbon nitride H-g-C 3 N 4 , B-g-C 3 N 4 , where the intermediate NH 2 -The preparation method of MIL-125 (Ti), comprising the following steps:
[0049] Step 1. Measure 1-8mL of methanol and 10-30mL of N,N-dimethylformamide solution into the container dropwise, stir for 10-60mins, then absorb 0.5-5mL of tetrabutyl titanate and slowly add into the container dropwise , and continue to stir for 20-40mins until the mixed solution is completely dispersed evenly, then add 0.5-5g of aminoterephthalic acid into the mixed solution, and continue to stir for 20-40mins until the solution is evenly mixed;
[0050] Step 2. Transfer the homogeneous solution obtained in step 1 to the lining of the polytetrafluoro...
Embodiment 1
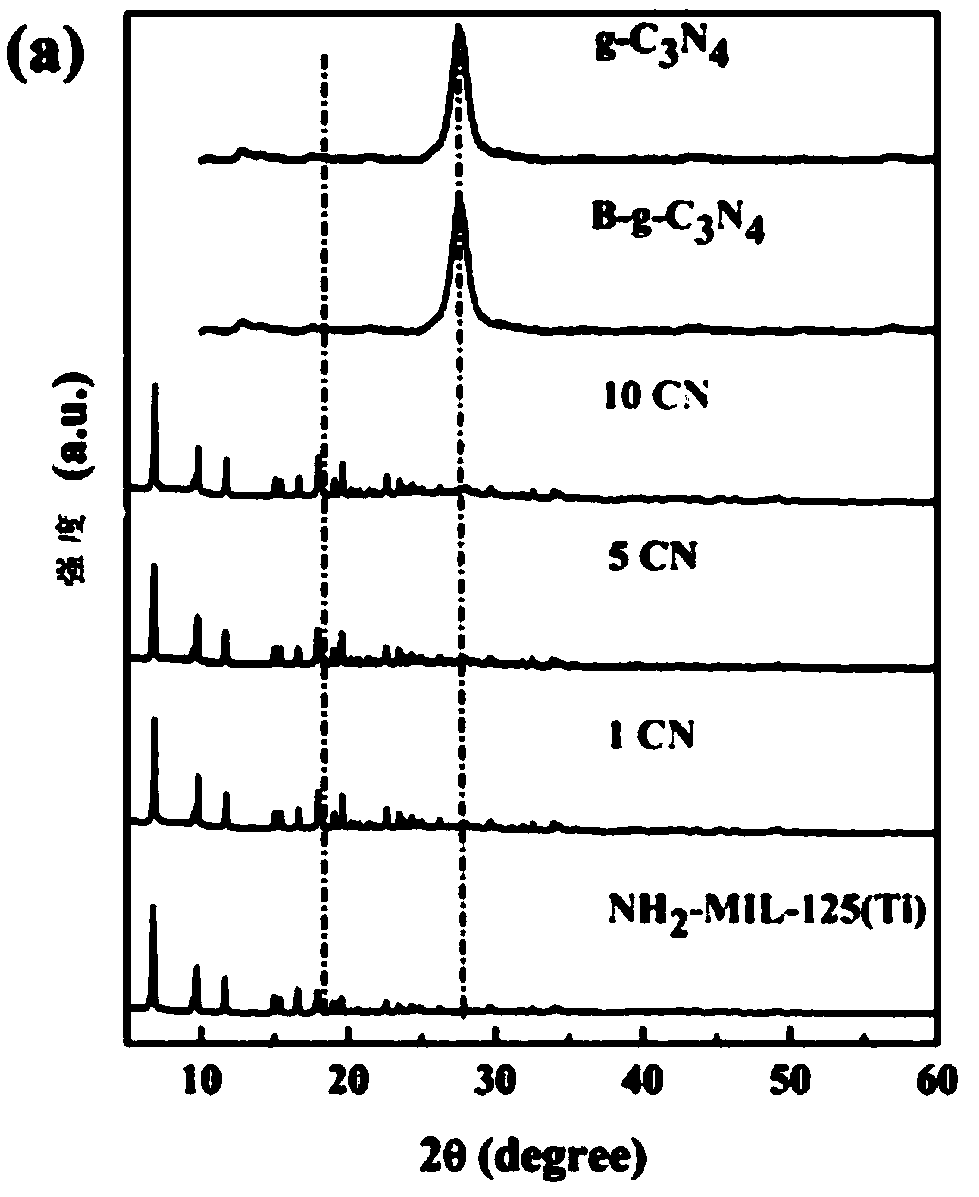
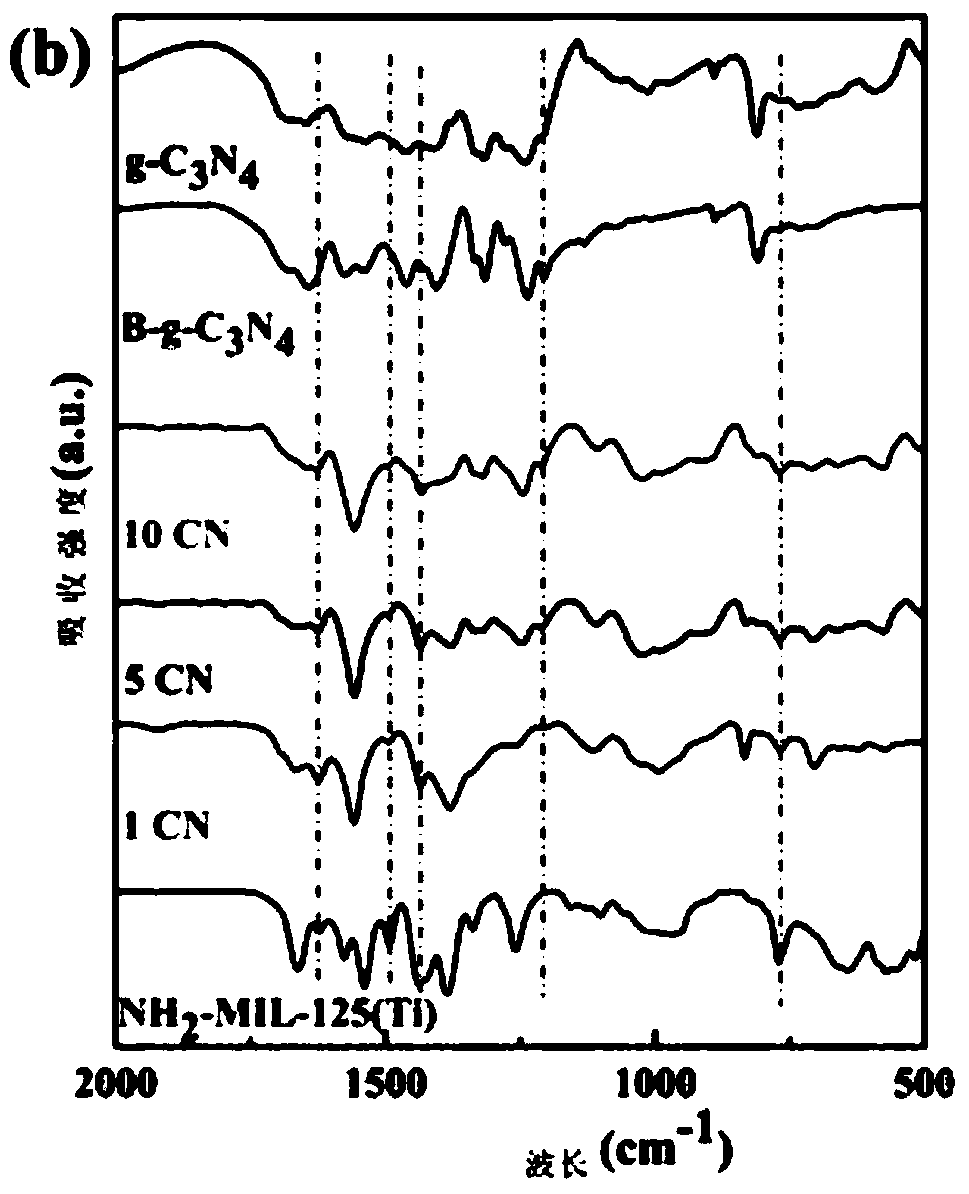
[0063] Example 1 prepares 1% g-C 3 N 4 / NH 2 -MIL-125(Ti) nanocomposite material
[0064] 1.1 Preparation of NH 2 -MIL-125(Ti)
[0065] Measure 2mL of methanol and 18mL of N,N-dimethylformamide solutions with a pipette and drop them into the beaker, stir for 30mins, then absorb 1.2mL of tetrabutyl titanate and slowly add them into the beaker drop by drop, and continue to stir for 30mins until The solution is completely dispersed evenly, then 1.1g of aminoterephthalic acid is added into the mixed solution, and the stirring is continued for 30mins until the solution is evenly dispersed.
[0066] Transfer the above homogeneously dispersed solution to the inner lining of a polytetrafluoroethylene reactor, conduct a solvothermal reaction at 150°C for 48 hours, take out the product after the reactor is cooled, and centrifuge with absolute ethanol and water at a speed of 8000r / mind respectively 6 times, then vacuum-dried at 60°C and taken out and ground into a uniform bright yello...
Embodiment 2
[0075] Embodiment 2 prepares 2.5% g-C 3 N 4 / NH 2 -MIL-125(Ti) nanocomposite material
[0076] First three steps are the same as 1.1~1.3 in embodiment 1
[0077] Step 2.4 Preparation of 5% g-C 3 N 4 / NH 2 -MIL-125(Ti) nanocomposite material
[0078] Take by weighing the NH obtained by 1.1 in 0.975g embodiment 1 2 -MIL-125 (Ti) and 0.025g B-g-C obtained in 1.3 of Example 1 3 N 4 Add it to a three-necked round bottom beaker, then add 20 mg of 1-ethyl-(2-dimethylaminopropyl) carbodiimide hydrochloride, 1-hydroxybenzotriazole and 300 μL of N, N-dipropylethyl amine, while feeding nitrogen into the three-necked round-bottom beaker while stirring, the stirring time is 48h, and then the product is filtered with DMF and a large amount of water, washed, and vacuum-dried to obtain 1% g-C 3 N 4 / NH 2 -MIL-125(Ti), referred to as 2.5CN.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com