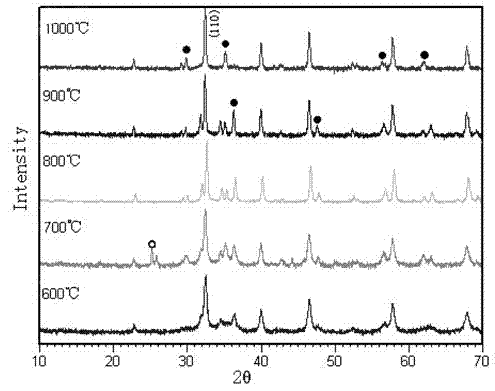
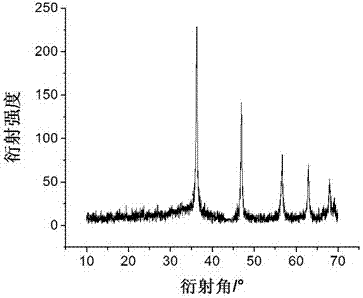
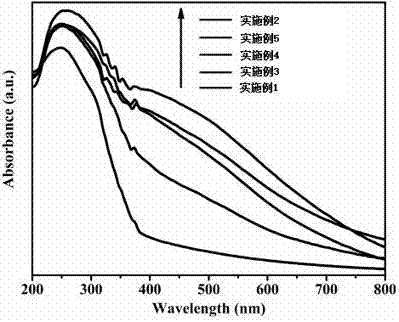
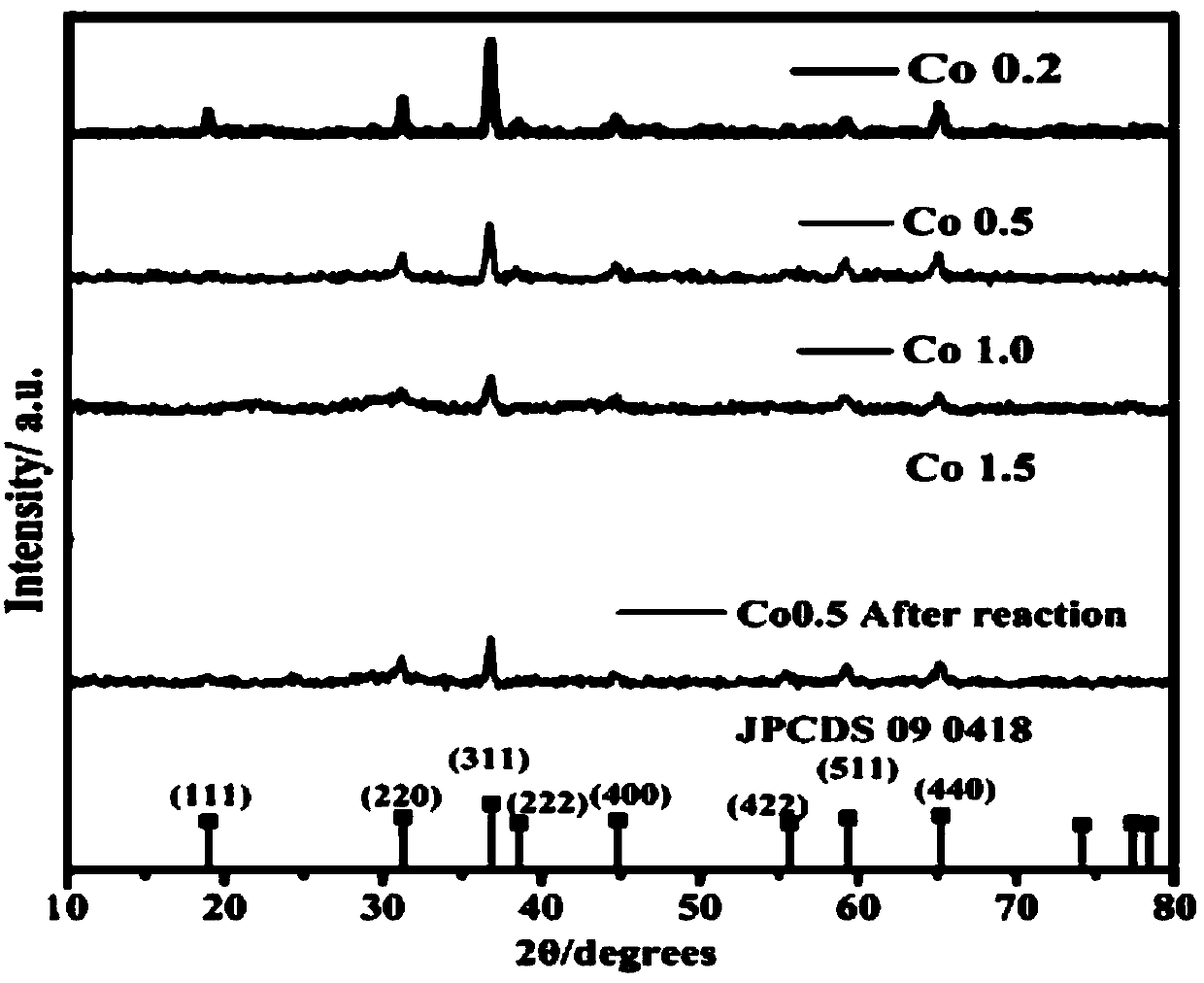
Patents
Literature
37results about How to "Suitable for expanding production requirements" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthesis and application of nitrogen-doped graphene quantum dot/similar-graphene phase carbon nitride composite material
InactiveCN105289689ABroaden the photoresponse rangePromote absorptionPhysical/chemical process catalystsHydrogen productionCvd grapheneCarbon composites
The invention discloses synthesis of nitrogen-doped graphene quantum dot / similar-graphene phase carbon nitride composite material and research and application of photocatalytic decomposition of aquatic hydrogen performance. A catalyst is composed of a nitrogen-doped graphene quantum dot and similar-graphene phase carbon nitride. Under simulated sunlight, the catalyst can efficiently and stably achieve water photolysis to produce hydrogen. The synthesis and application have the advantages that the catalyst is completely composed of nonmetal elements and has the advantages of being environmentally friendly and low in cost; the light response range of the similar-graphene phase carbon nitride is enlarged due to the doping of the nitrogen-doped graphene quantum dot, and absorption under the visible light is increased; the photosensitization effect and the ultrastrong electron conduction capacity of the nitrogen-doped graphene quantum dot are utilized at the same time, photo-induced electron and hole composition is restrained, and meanwhile the light utilization rate is improved; the raw materials are low in cost and easy to obtain, the synthesis method is simple, the synthesis yield and purity are high, and experimental repeatability is good.
Owner:NANCHANG HANGKONG UNIVERSITY
Controllable synthesis method of monoatomic catalyst with low cost and high loading capacity
ActiveCN111939961AIncrease loadThe content is easy to controlPhysical/chemical process catalystsHydrogen productionPhoto catalyticPtru catalyst
The invention discloses a controllable synthesis method of a monoatomic catalyst with low cost and high loading capacity, the monoatomic catalyst prepared by a method takes graphite-phase carbon nitride as a substrate, has the characteristics of low cost, high metal element loading capacity, controllable content and adjustable variety, and meanwhile, the method can be used for preparing the single-metal single-atom catalyst and a multi-metal single-atom catalyst, the single-atom catalyst has excellent catalytic activity and stability, and by taking the silver single-atom catalyst as an example, the visible light hydrogen production rate of the silver single-atom catalyst is 6.2 times that of silver nanoparticle-loaded graphite-phase carbon nitride, and after 60-hour continuous photocatalytic hydrogen production test, the hydrogen production rate is almost unchanged.
Owner:NANCHANG HANGKONG UNIVERSITY
Nano-composite material and preparation method thereof
InactiveCN109012607AEfficient separationImprove photocatalytic reduction of U(VI) to U(IV) performanceOther chemical processesOrganic-compounds/hydrides/coordination-complexes catalystsHeterojunctionUranyl
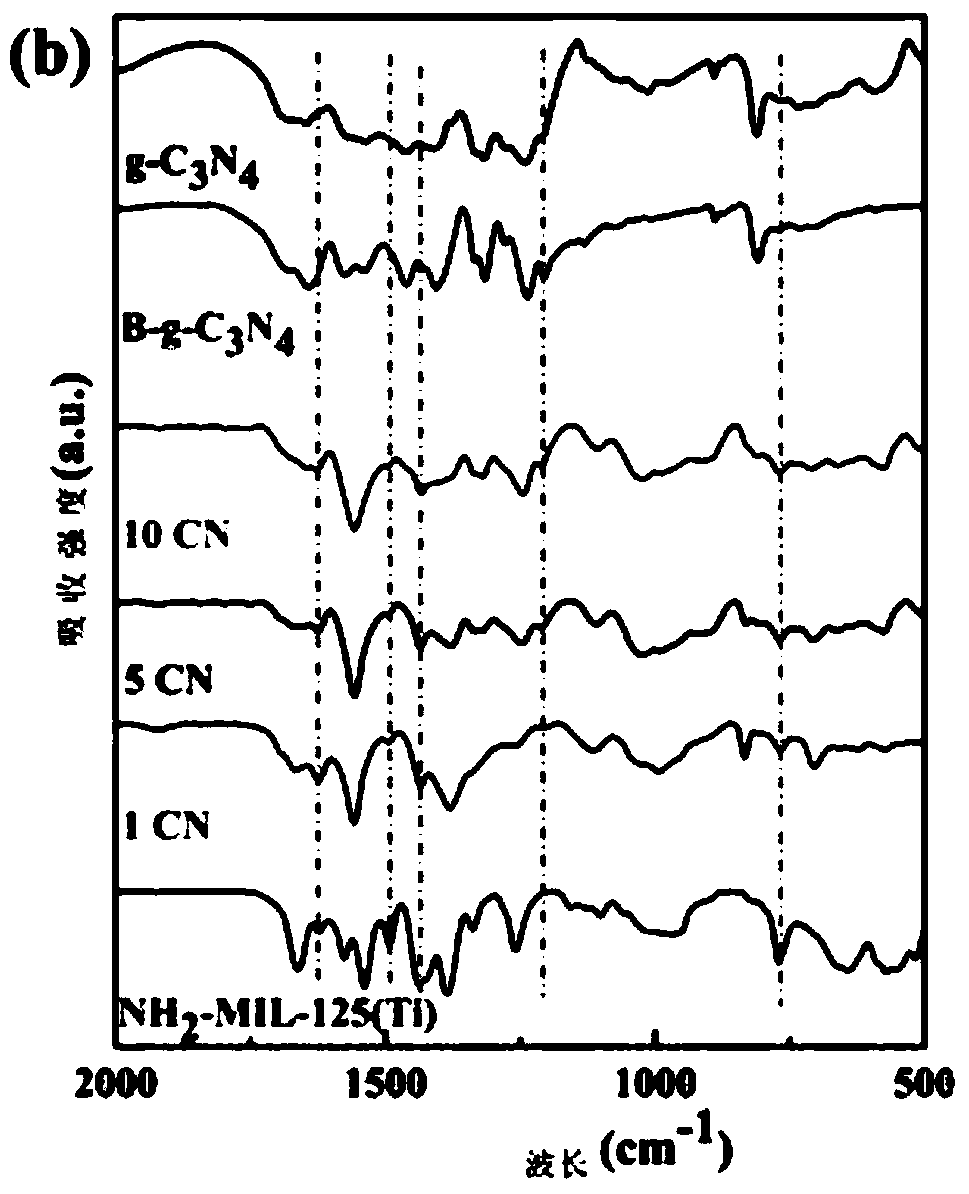
The invention discloses a g-C3N4 / NH2-MIL-125(Ti) nano-composite material and a preparation method thereof, and relates to the field of preparation of uranyl ion removal materials. The preparation method includes the following steps: preparing an intermediate NH2-MIL-125(Ti), preparing an intermediate H-g-C3N4, preparing an intermediate B-g-C3N4, and finally preparing the g-C3N4 / NH2-MIL-125(Ti) nano-composite material. The B-g-C3N4 is used for the first time, and the B-g-C3N4 and an aminated NH2-MIL-125(Ti) material undergo amido bond formation to synthesizes the heterojunction photocatalytic composite material, and photo-induced electrons and holes are effectively separated, so the performance for catalytic reduction of U (VI) into U(IV) is improved; and when the mass proportion of the B-g-C3N4 is 1%, the composite material can effectively remove radioactive uranyl ions through the synergistic mechanism of adsorption and photocatalysis, so the U (VI) removal rate is improved.
Owner:南通寰宇博新化工环保科技有限公司
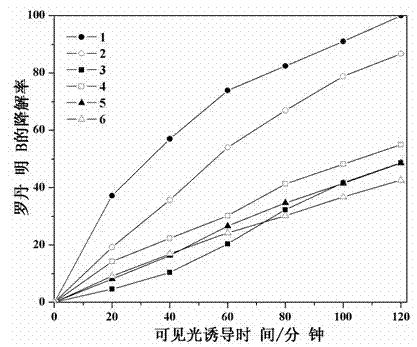
Catalyst for degrading rhodamine B by photocatalysis, and preparation method thereof
InactiveCN102357360AGood repeatabilityImprove thermal stabilityWater/sewage treatment by irradiationWater contaminantsStrong acidsThermal stability
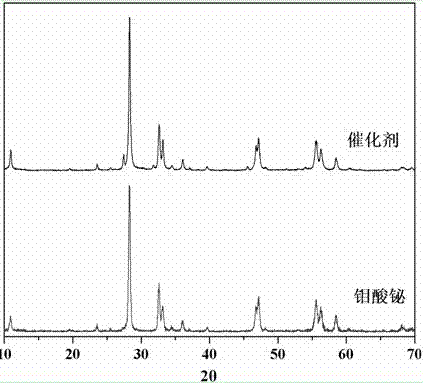
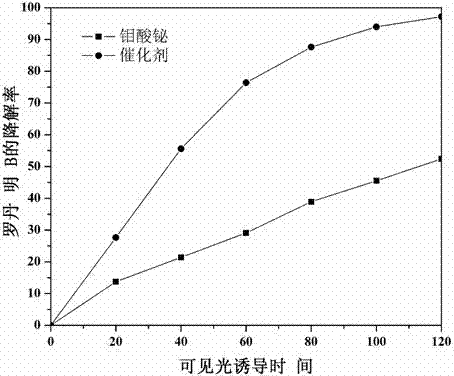
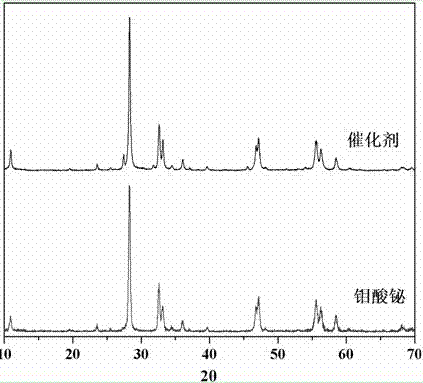
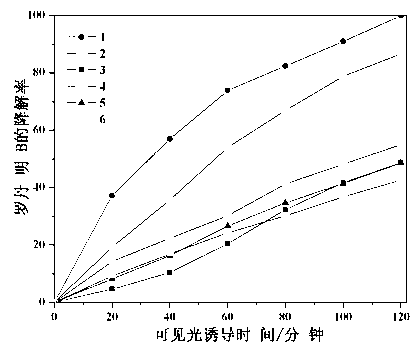
The invention relates to a catalyst for degrading rhodamine B by photocatalysis, and a preparation method thereof. The catalyst is prepared from TiO2-supported Bi2MoO6, wherein a molar ratio of the TiO2 to the Bi2MoO6 is 10%. With the induction of visible light, 100 ml of the rhodamine B with the concentration of 10<-5> mol / L can be completely degraded in 2 hours with 0.1 g of the catalyst. According to the present invention: 1, the catalyst of the present invention is prepared by a hydrothermal method, the operation is simple, the production cost is low, the synthesis yield is high, the purity is high, the reproducibility is good, and the method is applicable for the requirements of expanded production; 2, the catalyst of the present invention has characteristics of good thermal stability, strong acid resistance and strong alkali resistance; 3, the catalyst of the present invention has a good effect of photocatalytic degradation of organic materials, and the catalytic efficiency of the catalyst of the present invention is improved by 2 times than the catalytic efficiency of the single Bi2MoO6.
Owner:NANCHANG HANGKONG UNIVERSITY
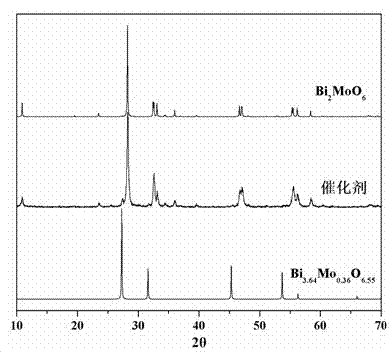
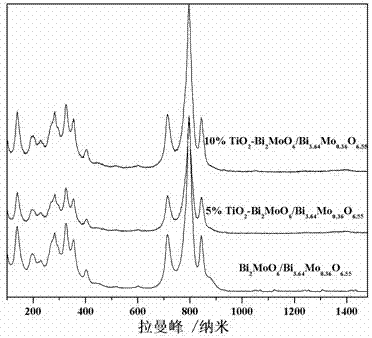
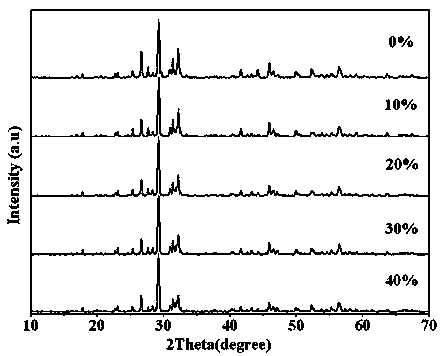
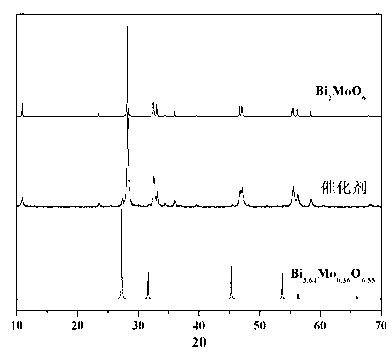
Ternary neterogeny structural light degradation organic matter catalyst TiO2-Bi2MoO6/Bi3.64Mo0.36O6.55 and preparation method thereof
ActiveCN102500361AGood repeatabilityImprove thermal stabilityWater/sewage treatment by irradiationCatalyst activation/preparationOrganic matterThermal stability
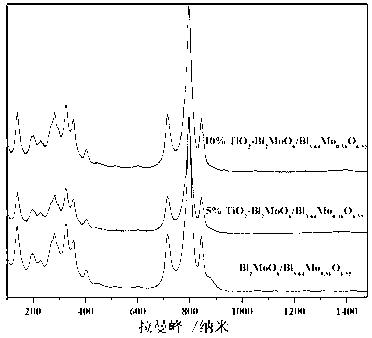
Disclosed is ternary neterogeny structural light degradation organic matter catalyst 5% TiO2-Bi2MoO6 / Bi3.64Mo0.36O6.55, which consists titanium dioxide (TiO2) and two types of dibismuth trimolybdenum dodecaoxide (Bi2MoO6 and Bi3.64Mo0.36O6.55) with different structures, wherein the titanium dioxide is 5% of total mole number of bismuth elements. The ternary neterogeny structural light degradationorganic matter catalyst 5% TiO2-Bi2MoO6 / Bi3.64Mo0.36O6.55 has the advantages that the catalyst is the ternary neterogeny structural light degradation organic matter catalyst reported for the first time; 2, the catalyst is directly synthesized by a one-step hydrothermal method, is simple in operation, low in production cost, high in synthesis yield and purity, fine in repeatability, and meets requirements of batch production; 3, the catalyst is fine in thermal stability and is high in acid resistance and alkali resistance; and 4, the catalyst has a good photocatalytic degradation organic effect, and has a higher catalysis effect as compared with single titanium dioxide, dibismuth trimolybdenum dodecaoxide (Bi2MoO6 and Bi3.64Mo0.36O6.55) and an optional combination (TiO2-Bi2MoO6, TiO2-Bi3.64Mo0.36O6.55 and Bi2MoO6 / Bi3.64Mo0.36O6.55) among the single titanium dioxide and the dibismuth trimolybdenum dodecaoxide (Bi2MoO6 and Bi3.64Mo0.36O6.55).
Owner:NANCHANG HANGKONG UNIVERSITY
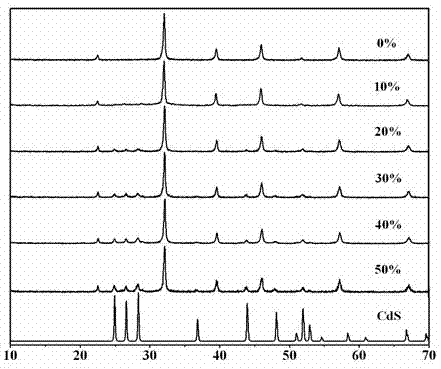
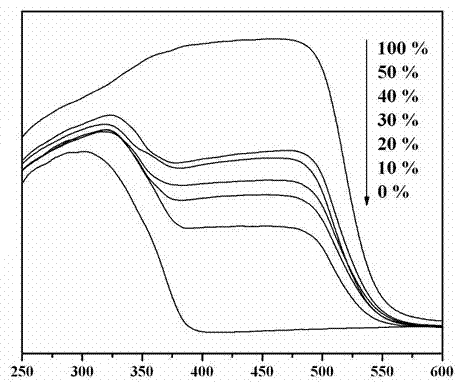
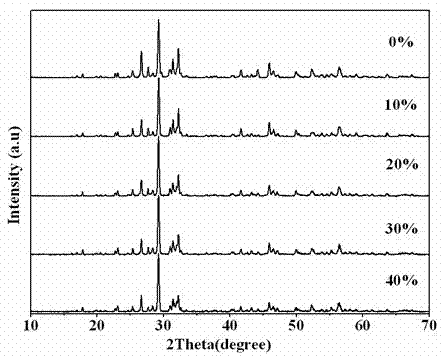
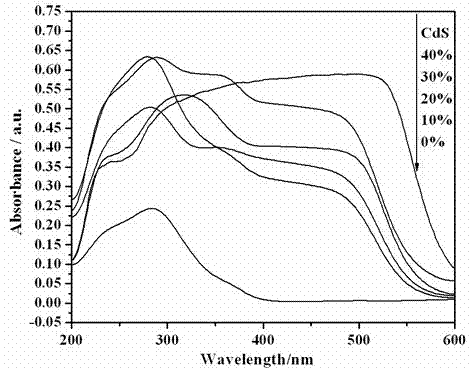
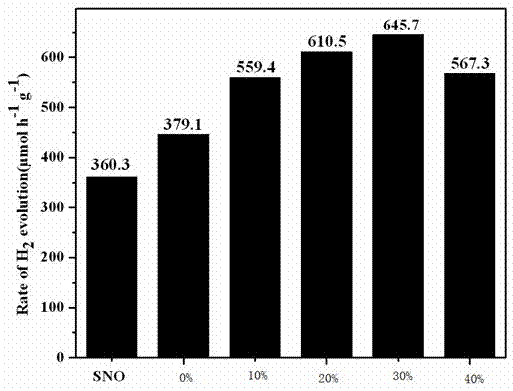
Photocatalytic water-splitting hydrogen production material CdS/Sr1.6Zn0.4Nb2O7 and preparation method thereof
ActiveCN103521244AEasy to operateHigh purityPhysical/chemical process catalystsHydrogen productionHeterojunctionPhotocatalytic water splitting
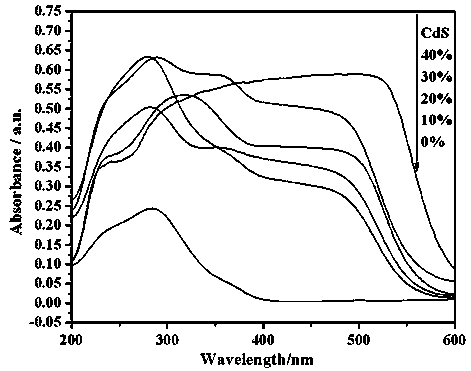
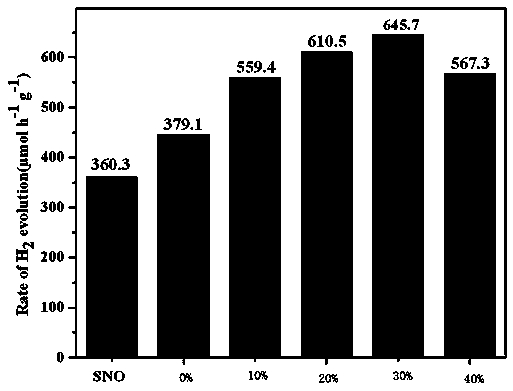
A photocatalytic decomposing water hydrogen production material CdS / Sr1.6Zn0.4Nb2O7 is a catalyst having a heterojunction structure formed by loading CdS on Sr1.6Zn0.4Nb2O7. The inventive catalyst is prepared by a hydrothermal method through sol gel. When a molar ratio of the CdS and the Sr1.6Zn0.4Nb2O7 is 3:7, the expression is 30%CdS / Sr1.6Zn0.4Nb2O7, and the catalytic effect of the material is best. Under the illumination of a 300 watt xenon lamp, Na2S / Na2SO3 is taken as a sacrifice reagent, the inventive material 30%CdS / Sr1.6Zn0.4Nb2O7 photocatalyzes and decomposes the water to produce the hydrogen without a co-catalyst of noble metal, and the efficiency is 645.7 mu mol.h<-1>.g<-1>. The photocatalytic decomposing water hydrogen production material of the invention has the advantages that: 1 the inventive catalyst is directly synthesized by the hydrothermal method through sol gel, the operation is simple, the production cost is low, the synthetic yield is higher, the purity is high and the repeatability is good, and the inventive catalyst is suitable for the demand of magnification production; 2, the inventive catalyst is good in the stability and is easy to reuse; and 3, the inventive catalyst has higher photocatalytic hydrogen production efficiency.
Owner:NANCHANG HANGKONG UNIVERSITY
Hydrogen material from photocatalytic water decomposition and preparation method thereof
InactiveCN102249296AEasy to operateReduce manufacturing costHydrogen productionTitanium compoundsChemistryDecomposition
The invention relates to a hydrogen material from photocatalytic water decomposition. The target compound is a perovskite structured material with a chemical formula of Sr2 / 3Zn1 / 3TiO3. Belonging to the cubic system, the compound has a space group of Pm-3m and the unit cell parameters of a=3.90-3.95??, b=3.90-3.95??, c=3.90-3.95??, a=89-91??, b=89-91??, g=89-91??, and Z=1. Induced by the ultraviolet light with a wavelength of 150-300 nanometers, and with ethanol as the sacrifice agent, the material of the invention can have high photocatalysis efficiency, and can reach a hydrogen production rate of 73.2??mol*h<-1>*g<-1> without a precious metal cocaralyst. The material of the invention has the advantages of: 1. simple sol-gel preparation method, low production cost, high synthetic yield, high purity and good repeatability, thus satisfying the requirements of expanded production; 2. good thermostability, strong acid and alkali resistance; 3. and good effect of hydrogen production from photocatalytic water decomposition.
Owner:NANCHANG HANGKONG UNIVERSITY
Preparation method of cerium oxide/graphene quantum dot/graphene-like carbon nitride composite photocatalytic material
InactiveCN107376976AInhibitory complexEfficient photocatalytic activityPhysical/chemical process catalystsElectron holeCarbon nitride
The invention provides a preparation method of a cerium oxide / graphene quantum dot / graphene-like carbon nitride composite photocatalytic material. The composite photocatalytic material is formed by inlaying cerium oxide in a graphene-like carbon nitride layered structure, and loading nitrogen-doped graphene quantum dots on the graphene-like carbon nitride. According to the preparation method of the cerium oxide / graphene quantum dot / graphene-like carbon nitride composite photocatalytic material, the photoresponse scope of the graphene-like carbon nitride is extended by doping the nitrogen-doped graphene quantum dots, and the composite photocatalytic material has high-efficiency photocatalysis activity by using rapid photo-generated electron-hole separating effect and electron transfer ability among the nitrogen-doped graphene quantum dots, the cerium oxide and the graphene-like carbon nitride.
Owner:吴滨
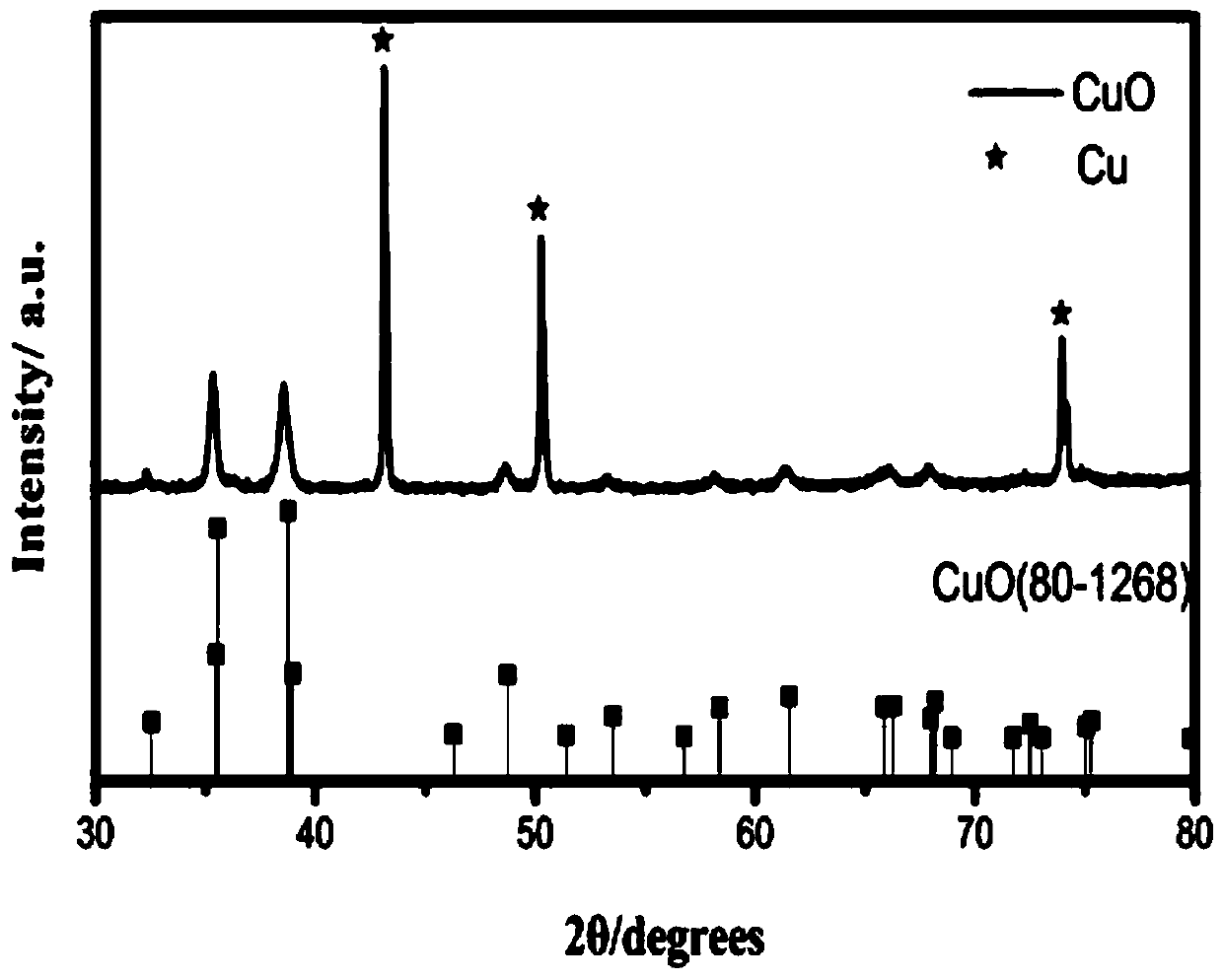
Electrocatalytic coupling advanced oxidation system
ActiveCN108033522AEfficient mineralizationEasy to recycleWater contaminantsWater/sewage treatment by oxidationHydrocarbonCarbon dioxide
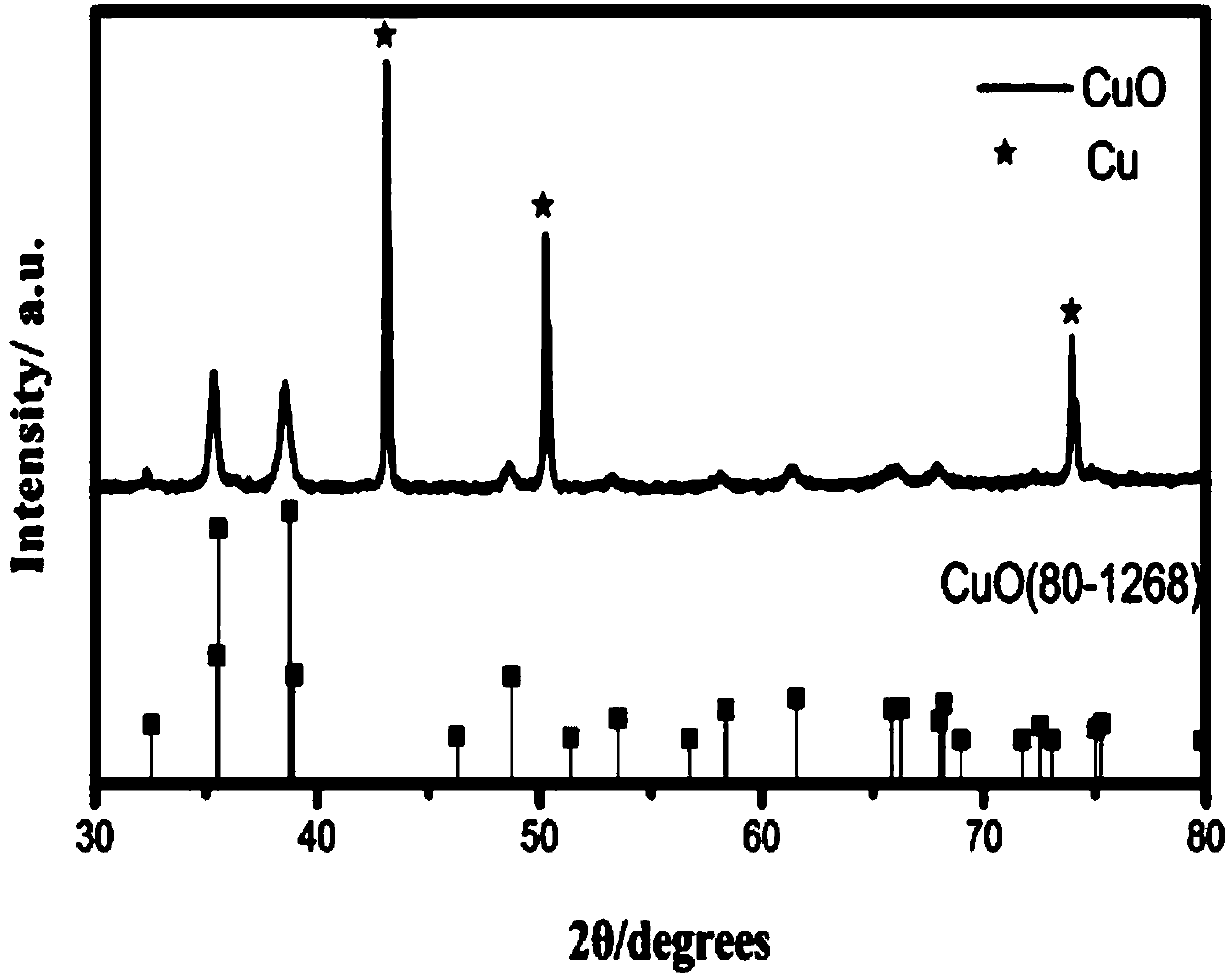
The invention provides an electrocatalytic coupling advanced oxidation system. The system can degrade organic pollutants into carbon dioxide and the carbon dioxide is synchronously electrocatalytically reduced to a hydrocarbon. In the catalytic system, three-dimensional hexagram-shaped Co3O4 and nano-sheet stack flower-shaped CuO are respectively used as an anode material and a cathode material, and the two electrode materials are prepared by a solvothermal method. In the three-electrode system electro-catalytic process, the Co3O4 electro-anode can thoroughly mineralize p-nitrophenol to CO2 and H2O, and the CuO electro-cathode can synchronously reduce the generated carbon dioxide into the useful hydrocarbon. When the additionally-applied bias voltage is 0.8V vs Ag / AgCl, the material has anoptimal catalytic effect, the removal rate of the p-nitrophenol can reach 98% or more, and the yields of methanol and ethanol reach 49.145 micromol / L / h and 20.475 micromol / L / h, respectively.
Owner:NANCHANG HANGKONG UNIVERSITY
Molybdenum disulfide/tin niobate composite nanomaterial and application thereof
ActiveCN107224986AEasy to operateReduce manufacturing costPhysical/chemical process catalystsHydrogen productionThioureaClean energy
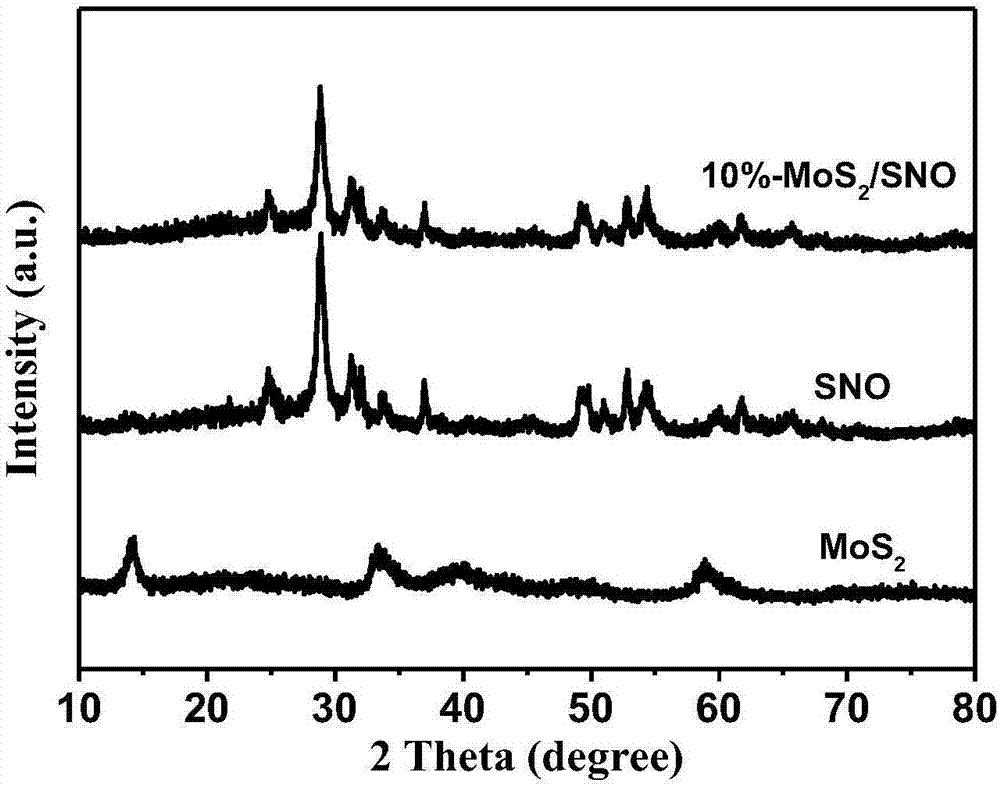
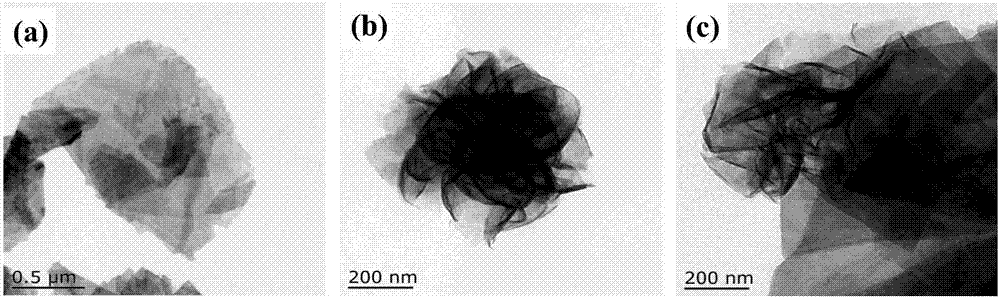
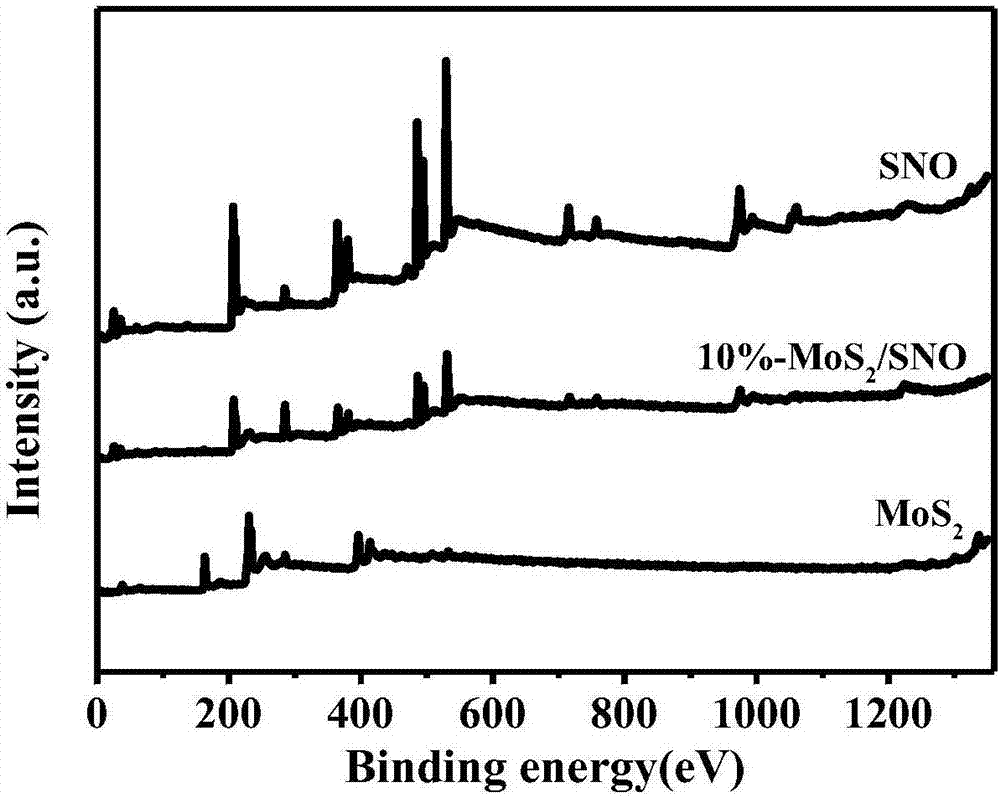
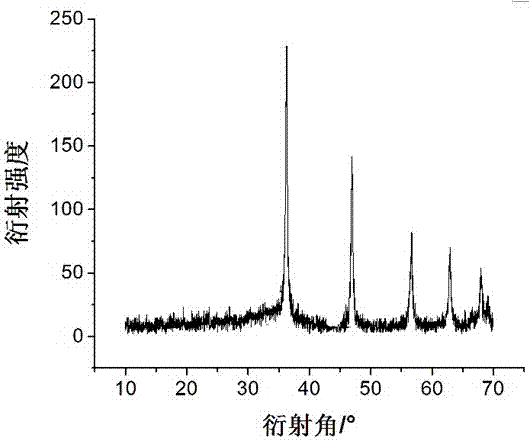
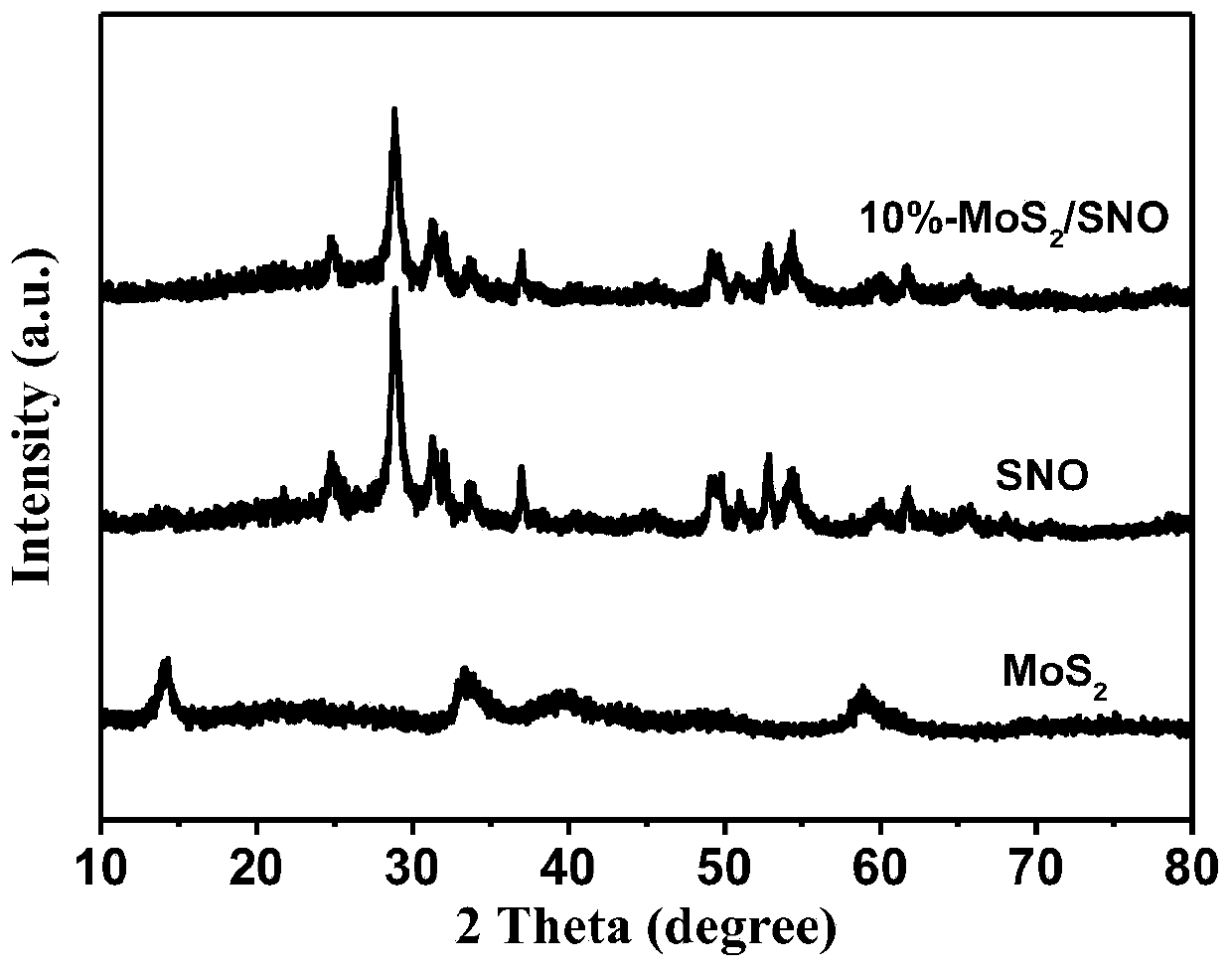
The invention aims at the problem of low catalytic efficiency of visible light catalysis of single two-dimensional molybdenum disulfide, provides a simple preparation method of molybdenum disulfide / tin niobate composite nanomaterial, is mainly used in the technology of hydrogen production realized through photocatalytic decomposition of water, and belongs to the technical field of composites and the field of clean energy. The MoS2 / SnNb2O6 composite is prepared by weighing and dissolving SnNb2O6 in deionized water, magnetically stirring for the first time, conducting ultrasonic dispersion, weighing sodium molybdate dihydrate and thiourea again, dissolving the sodium molybdate dihydrate and the thiourea into the solution, magnetically stirring for the second time, transferring a reaction liquid into a reaction kettle after the reactants are evenly mixed, putting the reaction kettle into an oven, conducting a hydrothermal reaction, centrifuging after naturally cooling to a room temperature, washing with water and washing with alcohol for a plurality of times, and drying, wherein a liner of the reaction kettle is made of teflon. The sheet MoS2 is dispersed on an SNO nanosheet.
Owner:JIANGSU UNIV
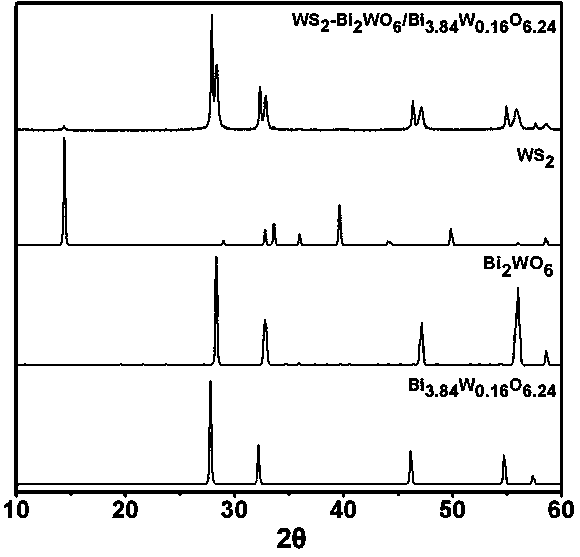
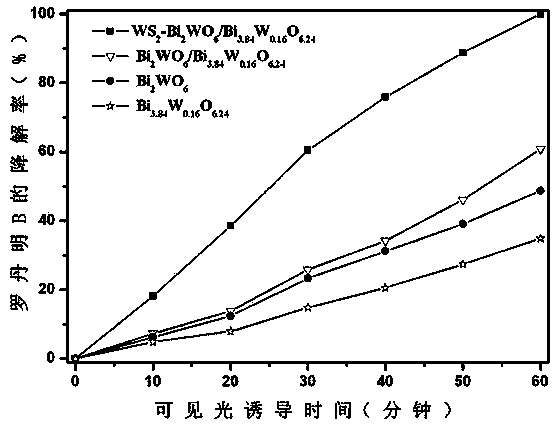
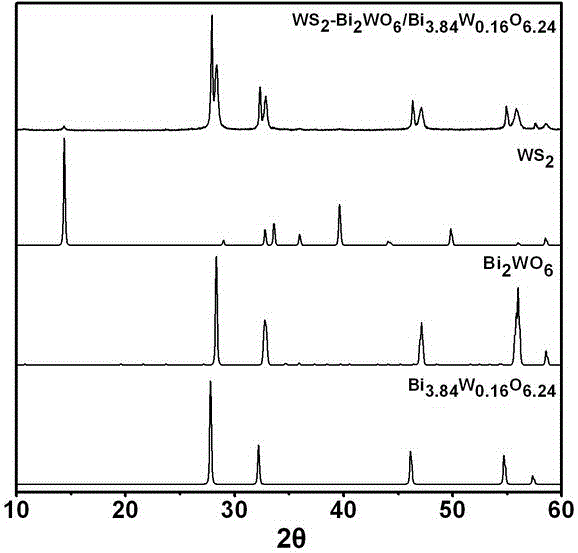
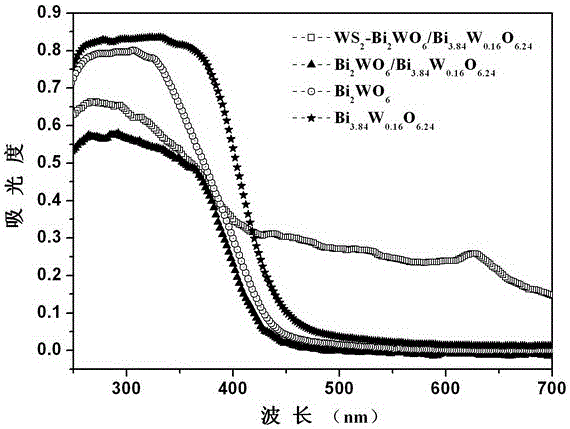
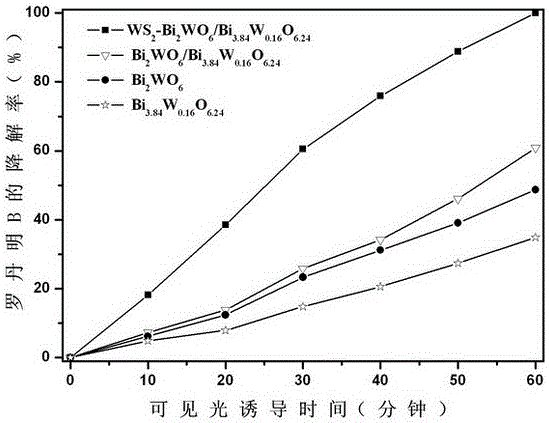
Ternary hetero-structured photo-degradation organic catalyst WS2-Bi2WO6/Bi3.84W0.16O6.24 and preparation method thereof
InactiveCN103962158AEasy to operateReduce manufacturing costPhysical/chemical process catalystsTungstateGraphene
The invention discloses ternary hetero-structured photo-degradation organic catalyst WS2-Bi2WO6 / Bi3.84W0.16O6.24 and a preparation method thereof. The ternary hetero-structured photo-degradation organic catalyst WS2-Bi2WO6 / Bi3.84W0.16O6.24 is characterized in that the catalyst is obtained through stratiform tungsten disulfide loaded on two bismuth tungstate of different structures, 100 ml of rhodamine B with the concentration of 10<-4>mol / L can be completely degraded by 0.1 g of the catalyst in one hour. According to the invention, the ternary hetero-structure photo-degradation organic catalyst WS2-Bi2WO6 / Bi3.84W0.16O6.24 and the preparation method thereof have the following advantages: 1. graphene type stratiform tungsten disulfide is firstly reported as the substrate materials of the catalysts, and the catalyst is a photo-degradation organic catalyst that has a ternary hetero-structure; 2. the catalyst is directly synthesized by adopting the single-step hydrothermal method, and is simple in operation, low in production cost, high in synthetic yield and purity, good in repeatability and suitable for requirements of expanded production.
Owner:NANCHANG HANGKONG UNIVERSITY
Electrocatalysis coupled advanced oxidation system and application thereof
ActiveCN110526343AImprove stabilityEfficient recyclingWater contaminantsWater/sewage treatment using germicide/oligodynamic-processElectricityPotassium persulfate
The invention provides an electrocatalysis coupled advanced oxidation system and an application thereof, and belongs to the technical field of sewage treatment. According to the electrocatalysis coupled advanced oxidation system, advanced oxidation and electrocatalysis are coupled on an anode, and a three-dimensional hexagonal star-shaped Co3O4 anode material can activate potassium persulfate to generate sulfate free radicals, can also electrocatalytically oxidize chloride ions to generate hypochlorous acid active substances, and can electrocatalytically catalyze an oxidation reaction of chloride ions and potassium persulfate to generate hypochlorous acid at the same time, so that organic pollutants can be efficiently and rapidly mineralized into CO2 and H2O.
Owner:NANCHANG HANGKONG UNIVERSITY
Method for preparing few-layer MoS2 nanosheets
InactiveCN104609474AImprove photocatalytic activityHigh purityMaterial nanotechnologyMolybdenum sulfidesWater bathsFiltration membrane
The invention discloses a method for preparing few-layer MoS2 nanosheets. The method comprises the following steps: preparing 100ml of 3% ethanol solution and placing the ethanol solution in 250ml of a three-mouth flask, weighing 0.1g of MoS2 powder and pouring the MoS2 powder in the mixed solution; placing the three-mouth flask in a water bath and heating to flow back, and continuously and naturally cooling the three-mouth flask to a room temperature for 2-4 hours when a temperature achieves 75-85 DEG C; centrifuging the mixed solution for 20 minutes at a certain rotational speed, taking the supernatant, carrying out suction filtration by using an organic filtration membrane, and drying for 12 hours at 60 DEG C to obtain a target product. The method disclosed by the invention has the following advantages that the problem of difficult preparation for the MoS2 nanosheets is solved, and a foundation is laid for the application expansions of the MoS2 nanosheets in other fields; the few-layer MoS2 nanosheets disclosed by the invention are prepared by using a method of mixed solvent backflow stripping, and the method disclosed by the invention is simple to operate, low in production cost, high in product purity, good in repeatability, and suitable for the requirements of expanded production.
Owner:NANCHANG HANGKONG UNIVERSITY
Organic divalent nonlinear optical crystal material 4-((1,2,4-triazole)-1-group) benzoic acid and preparation method thereof
InactiveCN102703984AGood second-order nonlinear optical propertiesThe second-order nonlinear optical properties are easy toPolycrystalline material growthOrganic chemistryNonlinear optical crystalBenzoic acid
The invention relates to an organic divalent nonlinear optical crystal material 4-((1,2,4-triazole)-1-group) benzoic acid and a preparation method of the organic divalent nonlinear optical crystal material 4-((1,2,4-triazole)-1-group) benzoic acid. The chemical formula of the organic divalent nonlinear optical crystal material is C9H7N3O2, the organic divalent nonlinear optical crystal material belongs to a monoclinic system, the space group is Pn, and the unit cell parameters are as follows: alpha equals to 90 degrees, beta equals to 93-96 degrees, gamma equals to 90 degrees, and z equals to 2. The advantages are as follows: 1, the organic compound crystal has excellent divalent nonlinear optical characteristics and the frequency-doubled effect of the organic compound crystal is 2.5 times that of an inorganic divalent nonlinear optical crystal monopotassium phosphate (KDP for short) with wide commercial application; and 2, the synthetic method is simple and easy to operate, material sources are sufficient, the production cost is low, and the compound is high in yield of crystal synthesization, high in purity and good in repeatability, therefore the requirements on expanded production are met.
Owner:JIANGXI NORMAL UNIV
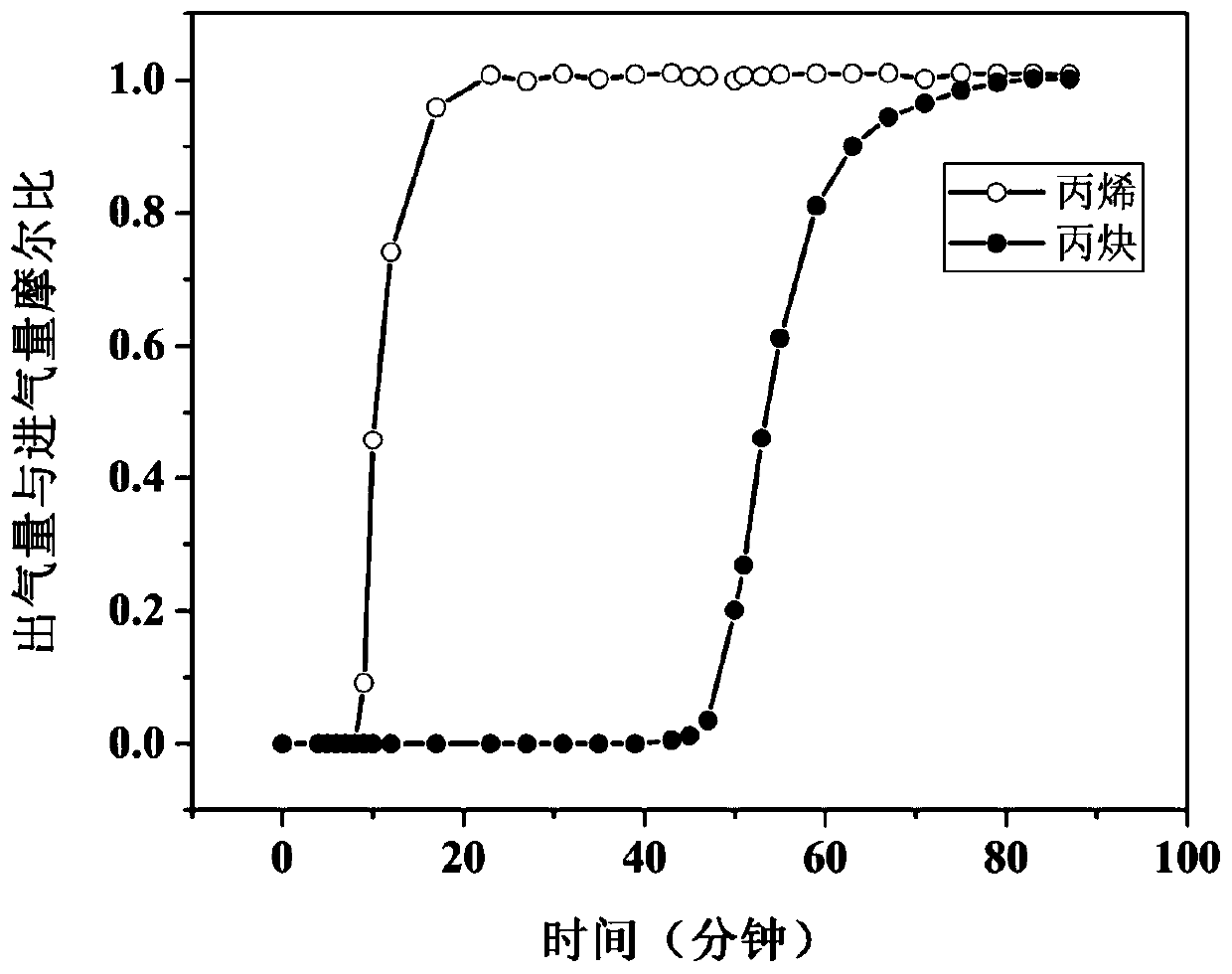
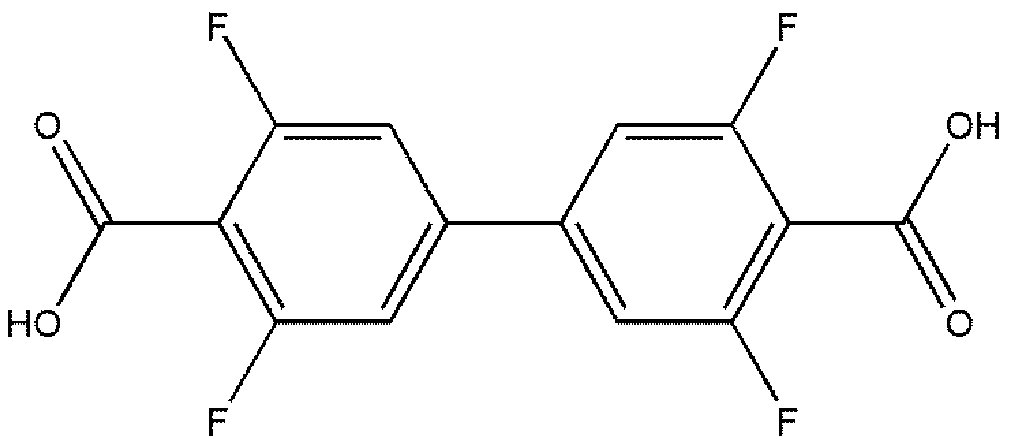
Terbium coordination polymer for separating propyne/propylene gas mixture and preparation method thereof
ActiveCN110698686AEasy to prepareEasy to operateOther chemical processesTetragonal crystal systemDicarboxylic acid
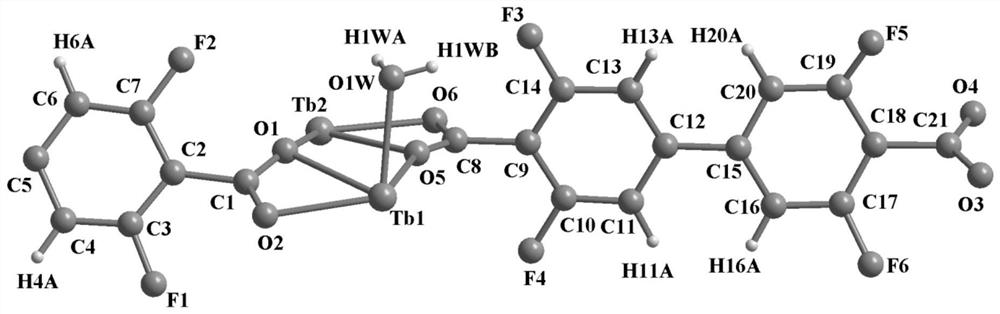
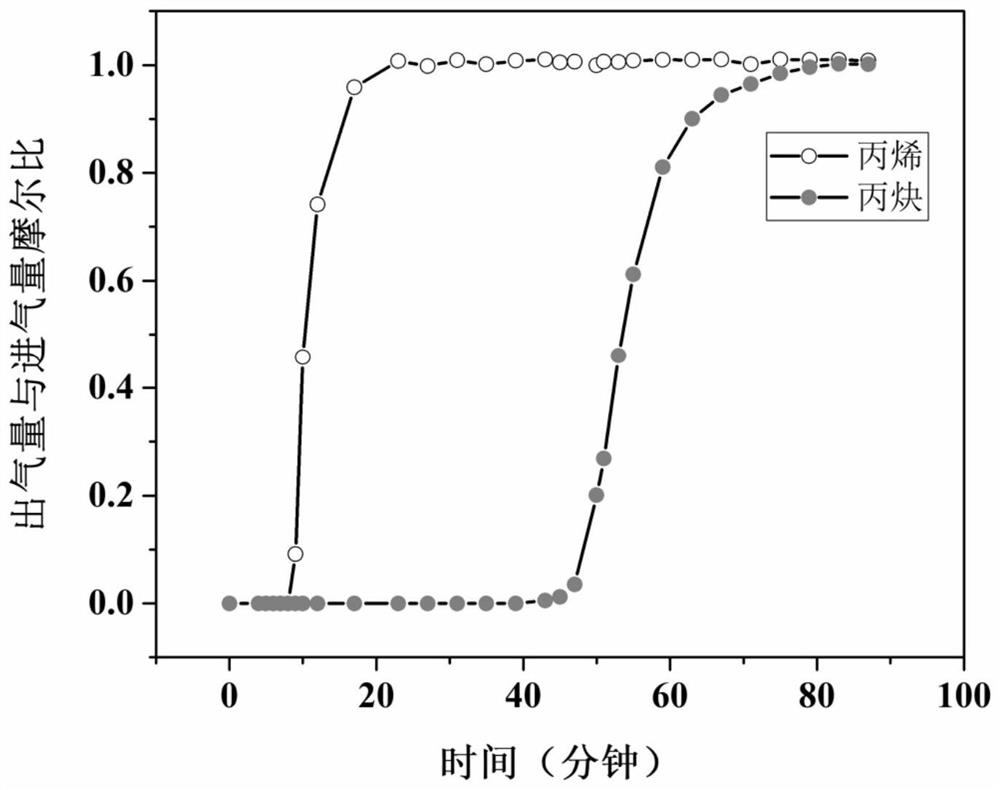
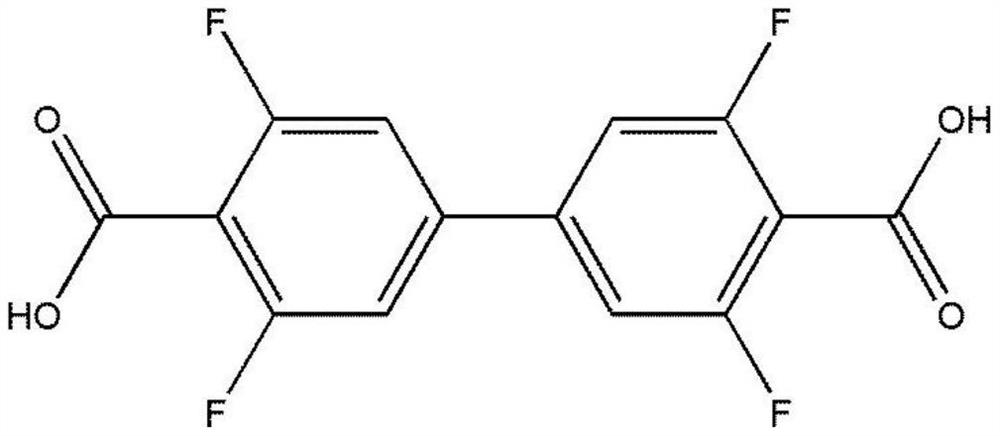
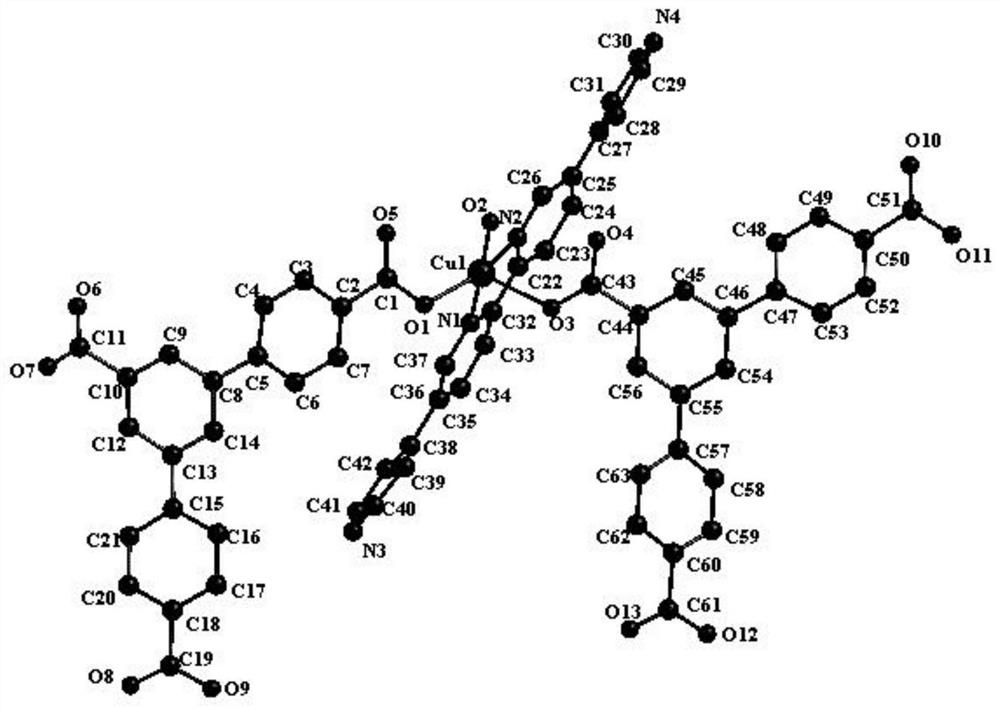
The invention provides a terbium coordination polymer for separating propyne / propylene gas mixture and a preparation method thereof. The terbium coordination polymer has a chemical formula of [Tb2. C42H12F12O12.H2O] and belongs to a tetragonal crystal system; the space group of the polymer is P4122; and unit cell parameters are that a is equal to 15.8-15.9 angstroms, b is equal to15.8-15.9 angstroms, c is equal to 27.5-27.6 angstroms, alpha is equal to 90 degrees, beta is equal to 90 degrees, gamma is equal to 90 degrees and Z is equal to 4. The preparation method of the terbium coordination polymer comprises the following steps: mixing a terbium salt, 3,3',5,5'-tetrafluorobiphenyl-4,4'-dicarboxylic acid and o-fluorobenzoic acid, then putting the formed mixture into a reaction kettle containing an organic solvent, and then carrying out a reaction at 358-373 K to obtain the terbium coordination polymer. The terbium coordination polymer can be used for separating the propargyl / propylenegas mixture.
Owner:JIANGXI NORMAL UNIVERSITY
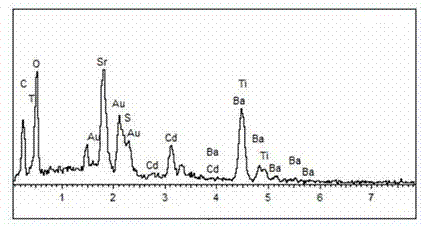
Material CdS/Ba0.4Sr0.6TiO3 for producing hydrogen by photocatalytically decomposing water and preparation method thereof
ActiveCN103586050AEasy to operateHigh purityPhysical/chemical process catalystsHydrogen productionCatalytic effectReagent
The invention relates to a material CdS / Ba0.4Sr0.6TiO3 for producing hydrogen by photocatalytically decomposing water, which is a heterostructure catalyst formed by supporting CdS onto Ba0.4Sr0.6TiO3. The catalyst is prepared by a one-step hydrothermal process. When the mole ratio of the CdS to the Ba0.4Sr0.6TiO3 is 0.4:1, the expression is 40%CdS / Ba0.4Sr0.6TiO3, and the material has the optimal catalytic effect. Under simulated sunlight, by using Na2S / Na2SO3 as a sacrifice reagent, the hydrogen production efficiency by photocatalytically decomposing water of the 40%CdS / Ba0.4Sr0.6TiO3 is up to 1268.4 mu mol.h<-1>.g<-1> under the condition of no noble metal for cocatalysis. The one-step hydrothermal process is directly used for synthesizing the catalyst, is simple to operate, has the advantages of low production cost, higher synthesis yield, higher purity and favorable repetitiveness, and is suitable for the requirement for scale-up production; the catalyst has high stability and is convenient for reutilization; and the catalyst has very high photocatalytic hydrogen production ratio under simulated sunlight.
Owner:NANCHANG HANGKONG UNIVERSITY
Preparation method and application of metal-organic microporous cobalt compound
InactiveCN110483588ASufficient sourceHigh yieldOrganic chemistry methodsCobalt organic compoundsSolventRaw material
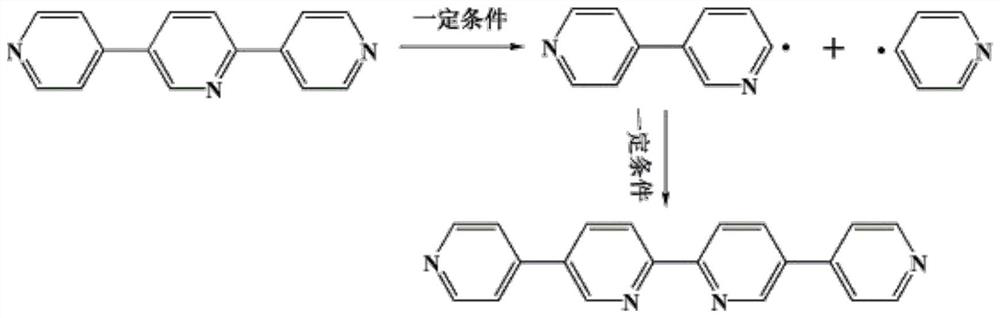
The invention discloses a preparation method and application of a metal-organic microporous cobalt compound, and relates to the technical field of coordination compounds; the metal-organic microporouscobalt compound is prepared from cobalt sulfate CoSO4, an organic ligand 2, 5-bis (4-pyridyl) pyrimidine and 1, 3, 5-tri (4-carboxyphenyl) benzene as raw materials by a hydrothermal synthesis methodin the presence of a reaction solvent; the compound 1 synthesized by the invention is of a two-dimensional microporous structure, has a plurality of active sites and is very novel in structure; the compound 1 with the active sites is combined with nanogold to modify a glassy carbon electrode. The modified glassy carbon electrode shows good selectivity, high stability and sensitivity and a wide detection range for detection of Pb < 2 + > ions.
Owner:WEST ANHUI UNIV
A ternary heterojunction photodegradation organic matter catalyst ws2-bi2wo6/bi3.84w0.16o6.24 and its preparation method
InactiveCN103962158BEasy to operateReduce manufacturing costPhysical/chemical process catalystsTungstateGraphene
The invention discloses ternary hetero-structured photo-degradation organic catalyst WS2-Bi2WO6 / Bi3.84W0.16O6.24 and a preparation method thereof. The ternary hetero-structured photo-degradation organic catalyst WS2-Bi2WO6 / Bi3.84W0.16O6.24 is characterized in that the catalyst is obtained through stratiform tungsten disulfide loaded on two bismuth tungstate of different structures, 100 ml of rhodamine B with the concentration of 10<-4>mol / L can be completely degraded by 0.1 g of the catalyst in one hour. According to the invention, the ternary hetero-structure photo-degradation organic catalyst WS2-Bi2WO6 / Bi3.84W0.16O6.24 and the preparation method thereof have the following advantages: 1. graphene type stratiform tungsten disulfide is firstly reported as the substrate materials of the catalysts, and the catalyst is a photo-degradation organic catalyst that has a ternary hetero-structure; 2. the catalyst is directly synthesized by adopting the single-step hydrothermal method, and is simple in operation, low in production cost, high in synthetic yield and purity, good in repeatability and suitable for requirements of expanded production.
Owner:NANCHANG HANGKONG UNIVERSITY
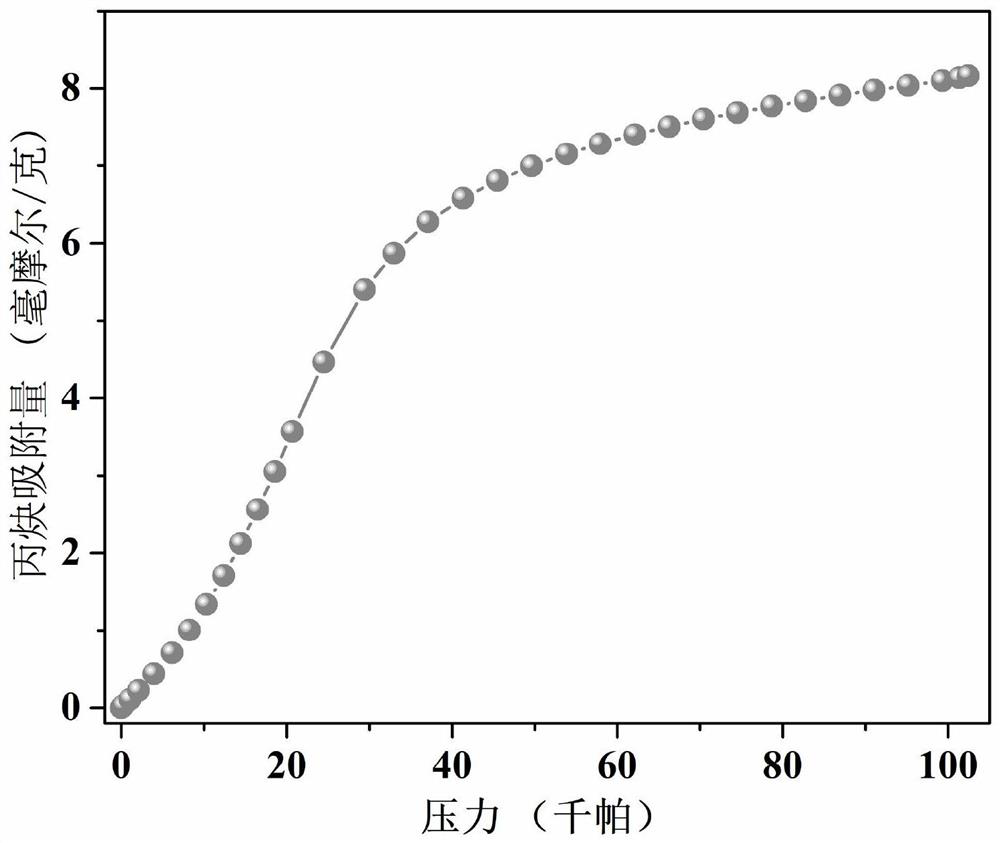
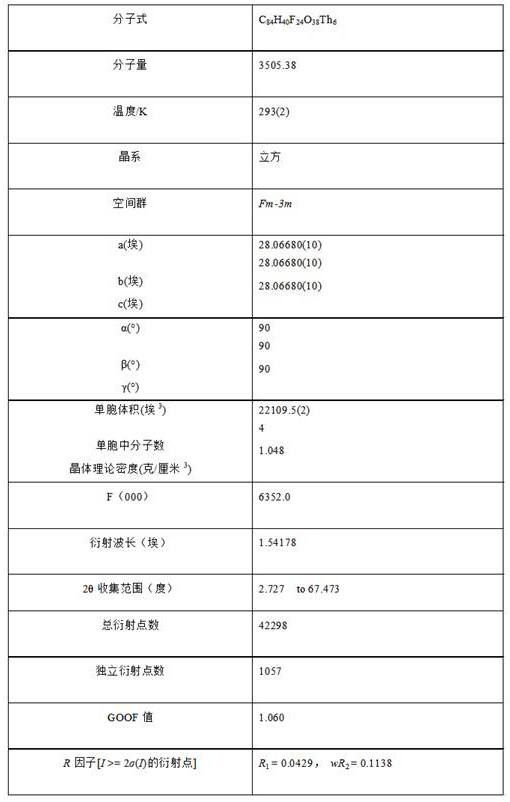
Thorium coordination polymer, its preparation method and its application in propyne storage
ActiveCN112661971BEasy to prepareEasy to operateOther chemical processesNuclear energy generationPolymer scienceCubic crystal system
The invention provides a thorium coordination polymer, its preparation method and its application in propyne storage. The chemical formula of the thorium coordination polymer is [Th 6 o 4 (OH) 4 (C 14 h 4 f 4 o 4 ) 6 (H 2 O) 6 ], C 14 h 4 f 4 o 4 It is the molecular formula of 3,3',5,5'-tetrafluorobiphenyl-4,4'-dicarboxylic acid with two carboxyl protons removed. It belongs to the cubic crystal system and the space group is Fm‑ 3m ; the unit cell parameter is a =28~28.1 Angstroms, b =28~28.1 Angstroms, c =28~28.1 Angstroms, =90º, =90º, =90º, Z =4. The preparation method of the thorium coordination polymer comprises the following steps: mixing thorium salt, 3,3',5,5'-tetrafluorobiphenyl-4,4'-dicarboxylic acid, nitric acid and solvent, and then transferring to a reaction kettle , and then react under the condition of 358~373 K to obtain the thorium coordination polymer. The thorium coordination polymer can be used to store propyne gas.
Owner:JIANGXI NORMAL UNIV
An Electrocatalytic Coupled Advanced Oxidation System
ActiveCN108033522BEasy to recycleImprove catalytic performanceWater contaminantsWater/sewage treatment by oxidationHydrocotyle bowlesioidesNitrobenzene
The invention provides an electrocatalytic coupling advanced oxidation system. The system can degrade organic pollutants into carbon dioxide and the carbon dioxide is synchronously electrocatalytically reduced to a hydrocarbon. In the catalytic system, three-dimensional hexagram-shaped Co3O4 and nano-sheet stack flower-shaped CuO are respectively used as an anode material and a cathode material, and the two electrode materials are prepared by a solvothermal method. In the three-electrode system electro-catalytic process, the Co3O4 electro-anode can thoroughly mineralize p-nitrophenol to CO2 and H2O, and the CuO electro-cathode can synchronously reduce the generated carbon dioxide into the useful hydrocarbon. When the additionally-applied bias voltage is 0.8V vs Ag / AgCl, the material has anoptimal catalytic effect, the removal rate of the p-nitrophenol can reach 98% or more, and the yields of methanol and ethanol reach 49.145 micromol / L / h and 20.475 micromol / L / h, respectively.
Owner:NANCHANG HANGKONG UNIVERSITY
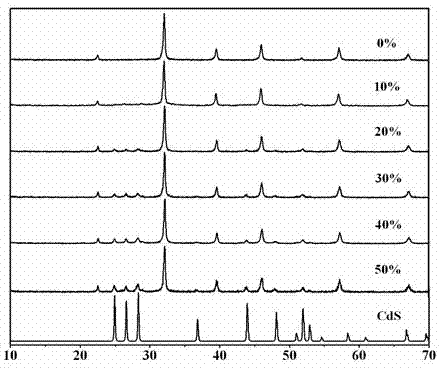
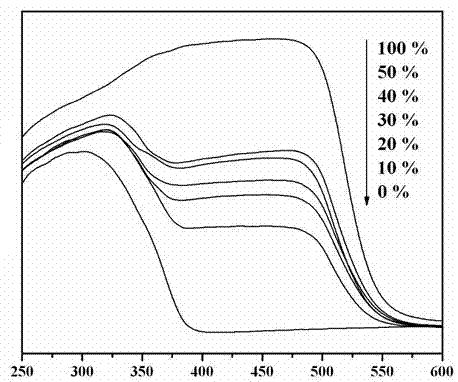
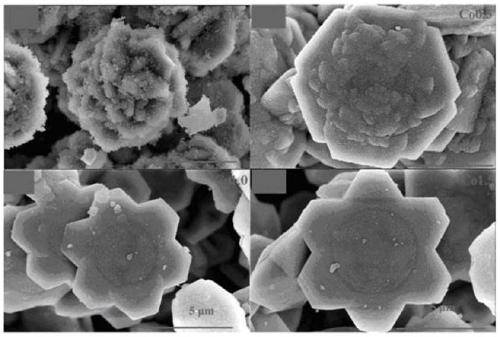
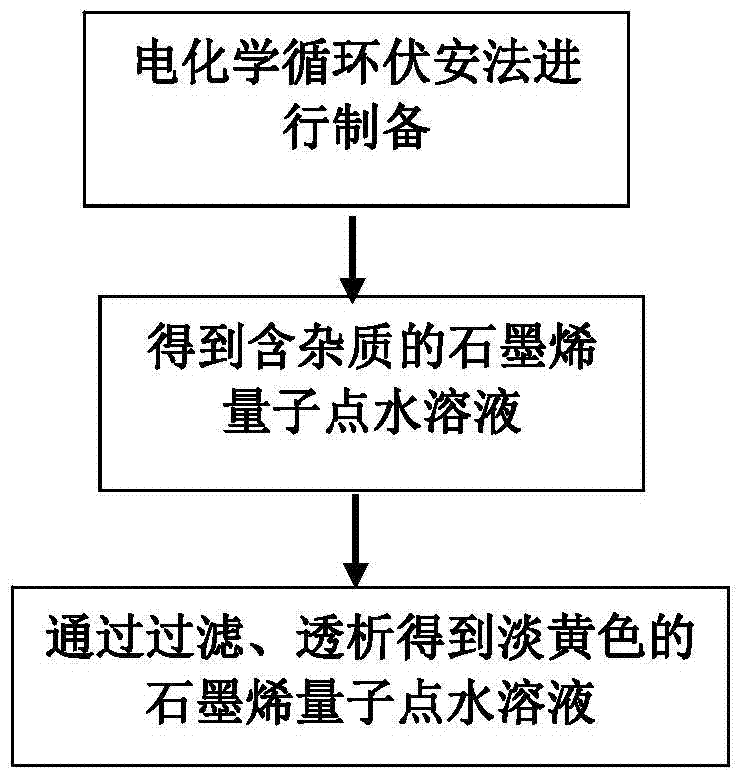
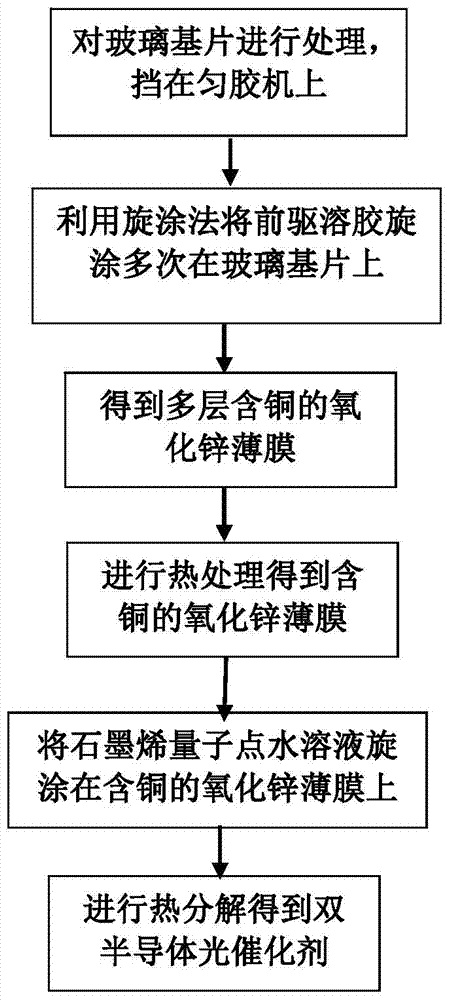
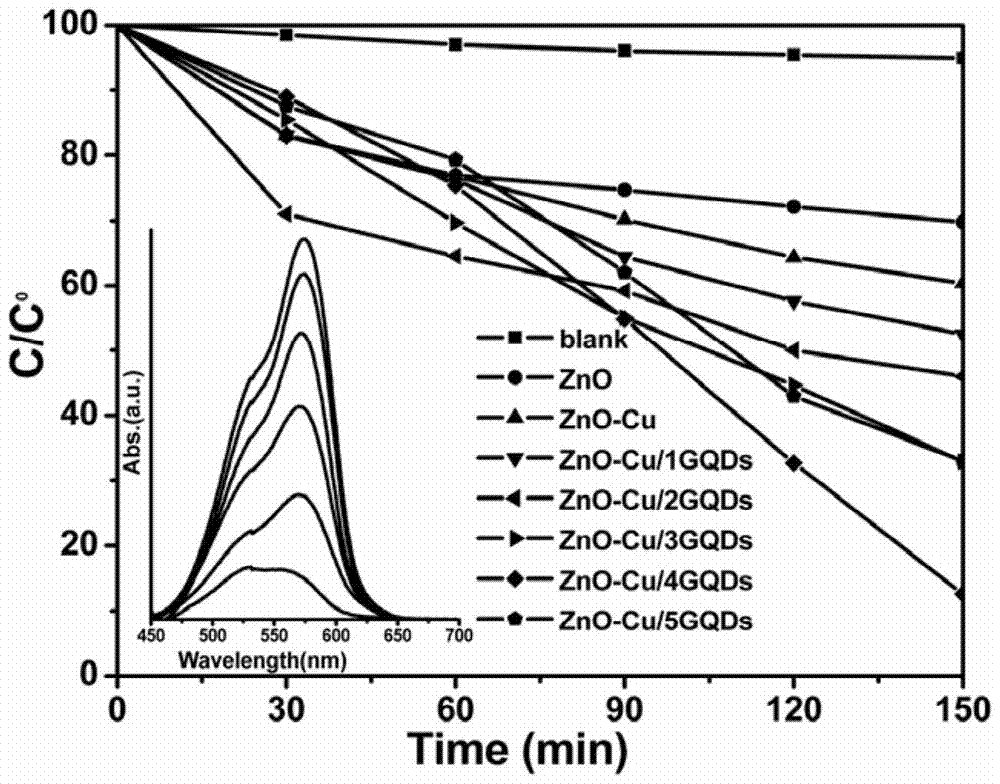
A kind of copper-containing zinc oxide/graphene quantum dot catalyst and preparation method thereof
InactiveCN104941651BPrevent photocorrosionPromote degradationWater/sewage treatment by irradiationWater contaminantsComposite filmUltraviolet lights
Owner:UNIV OF SCI & TECH BEIJING
Material CdS/Ba0.4Sr0.6TiO3 for producing hydrogen by photocatalytically decomposing water and preparation method thereof
ActiveCN103586050BEasy to operateHigh purityPhysical/chemical process catalystsHydrogen productionCatalytic effectReagent
The invention relates to a material CdS / Ba0.4Sr0.6TiO3 for producing hydrogen by photocatalytically decomposing water, which is a heterostructure catalyst formed by supporting CdS onto Ba0.4Sr0.6TiO3. The catalyst is prepared by a one-step hydrothermal process. When the mole ratio of the CdS to the Ba0.4Sr0.6TiO3 is 0.4:1, the expression is 40%CdS / Ba0.4Sr0.6TiO3, and the material has the optimal catalytic effect. Under simulated sunlight, by using Na2S / Na2SO3 as a sacrifice reagent, the hydrogen production efficiency by photocatalytically decomposing water of the 40%CdS / Ba0.4Sr0.6TiO3 is up to 1268.4 mu mol.h<-1>.g<-1> under the condition of no noble metal for cocatalysis. The one-step hydrothermal process is directly used for synthesizing the catalyst, is simple to operate, has the advantages of low production cost, higher synthesis yield, higher purity and favorable repetitiveness, and is suitable for the requirement for scale-up production; the catalyst has high stability and is convenient for reutilization; and the catalyst has very high photocatalytic hydrogen production ratio under simulated sunlight.
Owner:NANCHANG HANGKONG UNIVERSITY
Ternary heterostructure light degradation organic matter catalyst TiO2-Bi2MoO6/Bi3.64Mo0.36O6.55 and preparation method thereof
ActiveCN102500361BGood repeatabilityImprove thermal stabilityWater/sewage treatment by irradiationCatalyst activation/preparationOrganic matterThermal stability
Owner:NANCHANG HANGKONG UNIVERSITY
Terbium coordination polymer for separating propyne/propylene mixed gas and preparation method thereof
Owner:JIANGXI NORMAL UNIV
Synthesis of a copper compound and its application in photodegradation of organic dye methyl violet
ActiveCN113214492BImprove thermal stabilitySufficient sourceWater/sewage treatment by irradiationWater treatment compoundsMethyl violetCarboxyl radical
The invention discloses the synthesis of a copper compound and its application in the photodegradation of organic dye methyl violet. The steps of the preparation method are as follows: S1: 2,5-di(4-pyridyl)pyridine ligand and 1, Mixture of 3,5‑tris(4‑carboxyphenyl)benzene ligands, CuSO 4 Add to the reaction kettle, then add N,N-dimethylformamide, acetonitrile and deionized water, and adjust the pH of the solution to alkaline; S2: heat the adjusted mixed solution in S1, after the reaction The temperature was lowered to room temperature to obtain a copper compound. The compound prepared by the invention not only has good thermal stability, can exist stably in water with a pH of 3.0-11.5, and can photocatalytically degrade the organic dye methyl violet.
Owner:WEST ANHUI UNIV
A kind of cerium oxide/graphene quantum dot/graphene-like phase carbon nitride composite photocatalytic material and preparation method thereof
InactiveCN105396606BInhibitory complexEfficient photocatalytic activityPhysical/chemical process catalystsNitrogenCarbon nitride
Owner:青岛双创石墨烯科技有限公司
A kind of molybdenum disulfide/tin niobate composite nanomaterial and its application
ActiveCN107224986BEasy to operateReduce manufacturing costPhysical/chemical process catalystsHydrogen productionHydration reactionThiourea
Aiming at the problem of low visible light catalytic efficiency of single two-dimensional layered molybdenum disulfide, the present invention provides a simple preparation method of molybdenum disulfide / tin niobate composite nanomaterials, which is mainly used in the technology of photocatalytic water decomposition to produce hydrogen. , belongs to the field of composite materials technology and clean energy. Weigh SnNb 2 O 6 Dissolve in deionized water, stir with magnetic force for the first time, and disperse with ultrasonic. Then weigh sodium molybdate dihydrate and thiourea and dissolve in the above solution. Stir with magnetic force for the second time. After the reactants are evenly mixed, transfer the reaction solution to Put it into a reaction kettle lined with polytetrafluoroethylene and put it in an oven for hydrothermal reaction. After naturally cooling to room temperature, centrifuge, wash with water and alcohol several times, and dry to obtain the MoS. 2 / SnNb 2 O 6 Composite materials; sheet-like MoS 2 dispersed on SNO nanosheets.
Owner:JIANGSU UNIV
Photocatalytic water splitting hydrogen production material CdS/Ba0.9Zn0.1TiO3 and preparation method thereof
InactiveCN103381367BEasy to operateHigh purityPhysical/chemical process catalystsHydrogen productionPhotocatalytic water splittingCatalytic effect
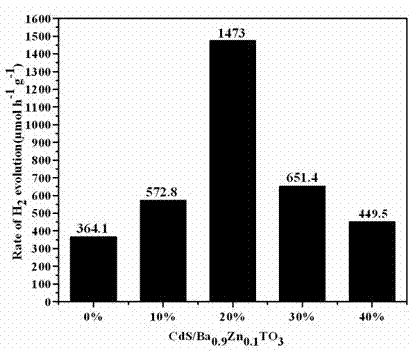
A photocatalytic water splitting hydrogen production material CdS / Ba0.9Zn0.1TiO3 is a catalyst with a heterostructure formed by enabling CdS to be loaded to Ba0.9Zn0.1TiO3 , and the catalyst is obtained by a hydrothermal sol-gel process. When the molar ratio of CdS and Ba0.9Zn0.1TiO3 is 1:5, the expression is 20%CdS / Ba0.9Zn0.1TiO3 , and the catalytic effect of the material is optimal. Under the exposure of a xenon lamp of 300 watts, Na2S / Na2SO3 serves as a sacrifice reagent. The photocatalytic water splitting hydrogen production material CdS / Ba0.9Zn0.1TiO3 has the advantages that the catalyst is directly synthesized in a hydrothermal sol-gel process and is simple in operate, low in production cost, high in synthesis yield, extremely high in purity, good in repeatability and suitable for the requirements of expanded production; the catalyst is good in stability and convenient to recycle; and the catalyst is high in photocatalysis hydrogen production efficiency.
Owner:NANCHANG HANGKONG UNIVERSITY
Photocatalytic water-splitting hydrogen production material CdS/Sr1.6Zn0.4Nb2O7 and preparation method thereof
ActiveCN103521244BEasy to operateHigh purityPhysical/chemical process catalystsHydrogen productionHeterojunctionPhotocatalytic water splitting
A photocatalytic decomposing water hydrogen production material CdS / Sr1.6Zn0.4Nb2O7 is a catalyst having a heterojunction structure formed by loading CdS on Sr1.6Zn0.4Nb2O7. The inventive catalyst is prepared by a hydrothermal method through sol gel. When a molar ratio of the CdS and the Sr1.6Zn0.4Nb2O7 is 3:7, the expression is 30%CdS / Sr1.6Zn0.4Nb2O7, and the catalytic effect of the material is best. Under the illumination of a 300 watt xenon lamp, Na2S / Na2SO3 is taken as a sacrifice reagent, the inventive material 30%CdS / Sr1.6Zn0.4Nb2O7 photocatalyzes and decomposes the water to produce the hydrogen without a co-catalyst of noble metal, and the efficiency is 645.7 mu mol.h<-1>.g<-1>. The photocatalytic decomposing water hydrogen production material of the invention has the advantages that: 1 the inventive catalyst is directly synthesized by the hydrothermal method through sol gel, the operation is simple, the production cost is low, the synthetic yield is higher, the purity is high and the repeatability is good, and the inventive catalyst is suitable for the demand of magnification production; 2, the inventive catalyst is good in the stability and is easy to reuse; and 3, the inventive catalyst has higher photocatalytic hydrogen production efficiency.
Owner:NANCHANG HANGKONG UNIVERSITY
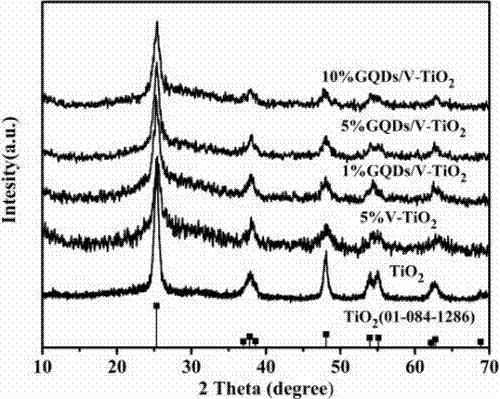
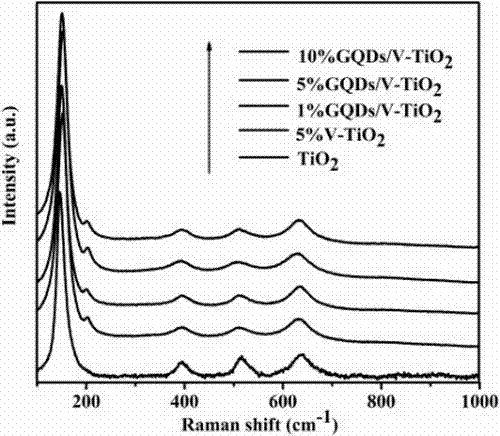
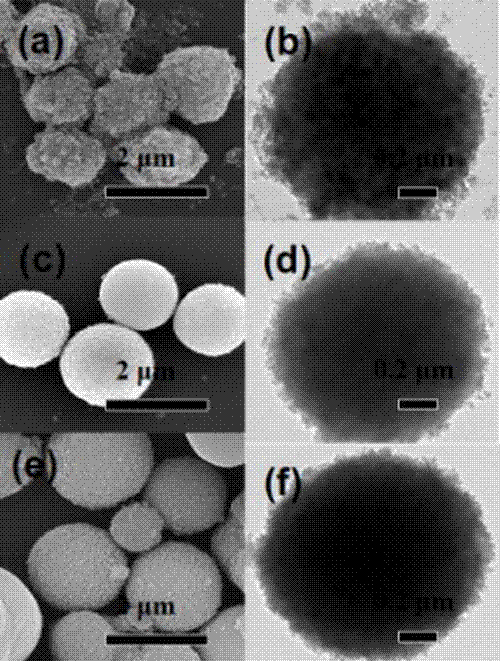
Preparation method of a graphene quantum dot/vanadium-doped mesoporous titanium dioxide composite photocatalyst
ActiveCN105195131BReduced band gapIncrease profitHydrocarbon from carbon oxidesMetal/metal-oxides/metal-hydroxide catalystsSynthesis methodsVanadium doping
The invention discloses a method for preparing a graphene quantum dot / vanadium-doped mesoporous titanium dioxide composite photocatalyst. The catalyst of the invention is composed of graphene quantum dots and vanadium-doped mesoporous titanium dioxide microspheres obtained by solvothermal heat. Under simulated sunlight, the catalyst not only effectively mineralized methylene blue into CO2 and H2O, but also reduced the carbon dioxide produced by its catalytic oxidation to useful hydrocarbons. The advantages of the present invention are: 1. The doping of vanadium reduces the bandgap width of titanium dioxide, thereby improving its response range under visible light; 2. At the same time, the photosensitization and super-strong electron conduction of graphene quantum dots are utilized ability, which not only inhibits the recombination of photogenerated electrons and holes, but also improves the utilization rate of light; 3, the material of the present invention is cheap and easy to obtain, the synthesis method is simple, the synthesis yield and purity are high, and the experiment repeatability is good, suitable for Expanded production requirements.
Owner:NANCHANG HANGKONG UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com