Electrocatalytic coupling advanced oxidation system
A technology of advanced oxidation and electrocatalysis, applied in the fields of oxidizing water/sewage treatment, chemical instruments and methods, water/sludge/sewage treatment, etc., can solve problems such as pollution, achieve good experimental repeatability, easy recovery, and cheap materials easy-to-get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Three-dimensional six-pointed star-shaped Co 3 o 4 Synthesis of Electrode Anode Materials:
[0033] Dissolve 2 mmoles of 0.582 grams of cobalt nitrate in 10 milliliters of ethylene glycol and 30 milliliters of water and stir. After completely dissolving, add 0.5 grams of urea and stir evenly, then add 0.05 grams of cetyltrimethylammonium bromide and 1.455 grams of fluoride ammonium, stirred at 25°C for 30 minutes to form a homogeneous, transparent, colorless solution, and the solution was transferred to a 100 ml polytetrafluoroethylene reactor lining. Then place a piece of cleaned conductive glass (1.5 cm × 4.0 cm) in the reaction kettle at an angle, with the conductive side of the conductive glass facing up, and finally transfer the reaction kettle to a drying oven at 120°C for reaction, and react for 12 hours to obtain a purple color on the conductive glass. precursors. Put its conductive glass into a muffle furnace, pre-calcined at 350°C for 2 hours, then calcined...
Embodiment 2
[0035] Synthesis of nanosheet-stacked flower-like CuO cathode: 6.4g of sodium hydroxide, 1.0954g of ammonium persulfate and 0.211g of sodium tungstate were dissolved in 32ml of deionized water and stirred at 25°C for 30 minutes to form a uniform, transparent, colorless solution. Then 0.4614 g of sodium lauryl sulfate was added to the above solution to form a uniform white solution. After the sodium lauryl sulfate is completely dissolved, move it into a polytetrafluoroethylene-lined stainless steel autoclave, and immerse the cleaned copper mesh (1.5 cm × 4.0 cm) in the above solution. The autoclave was closed and reacted at 130°C for 24 hours, then cooled to room temperature, the copper grid was taken out, and washed with deionized water to obtain a black sample CuO.
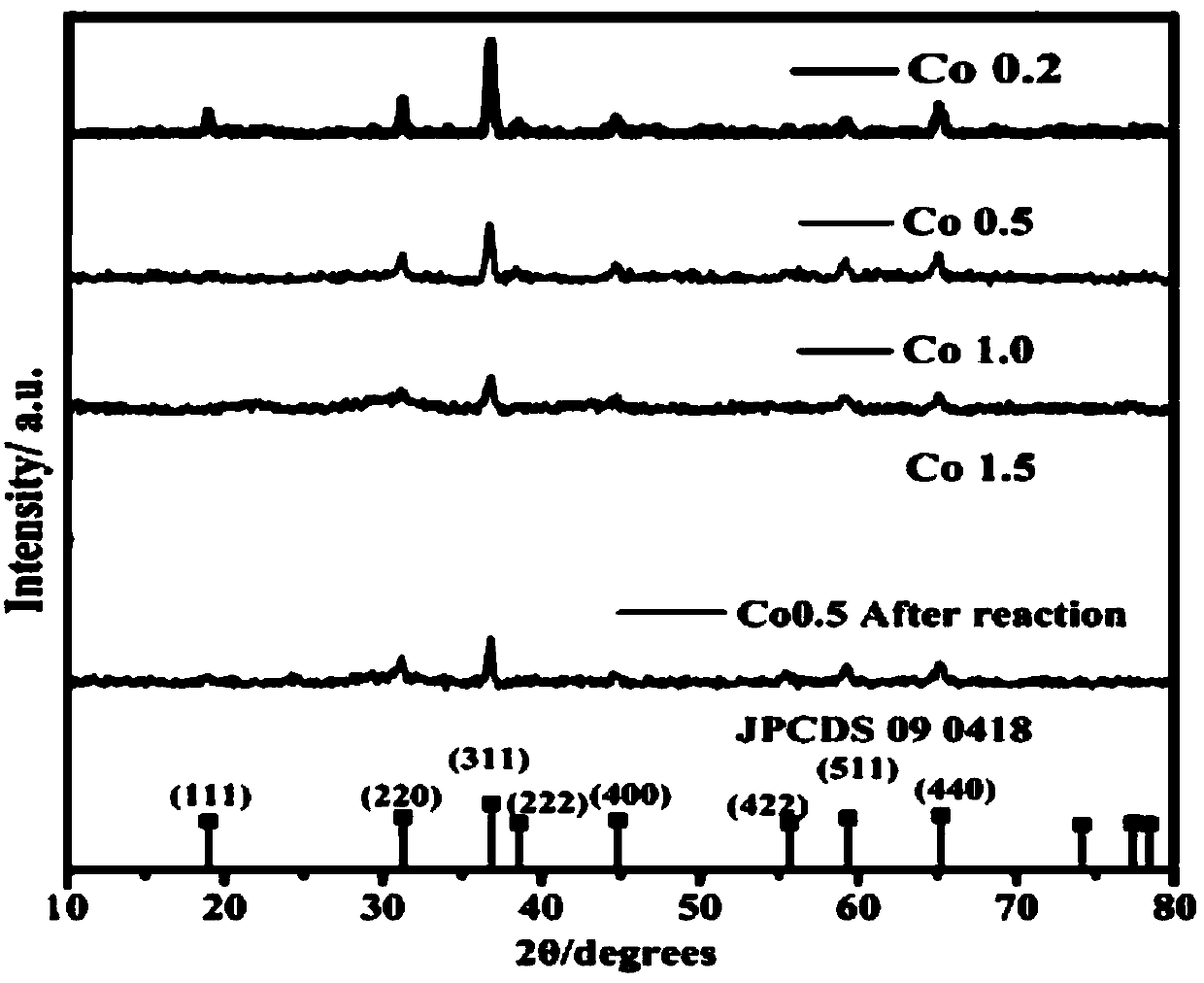
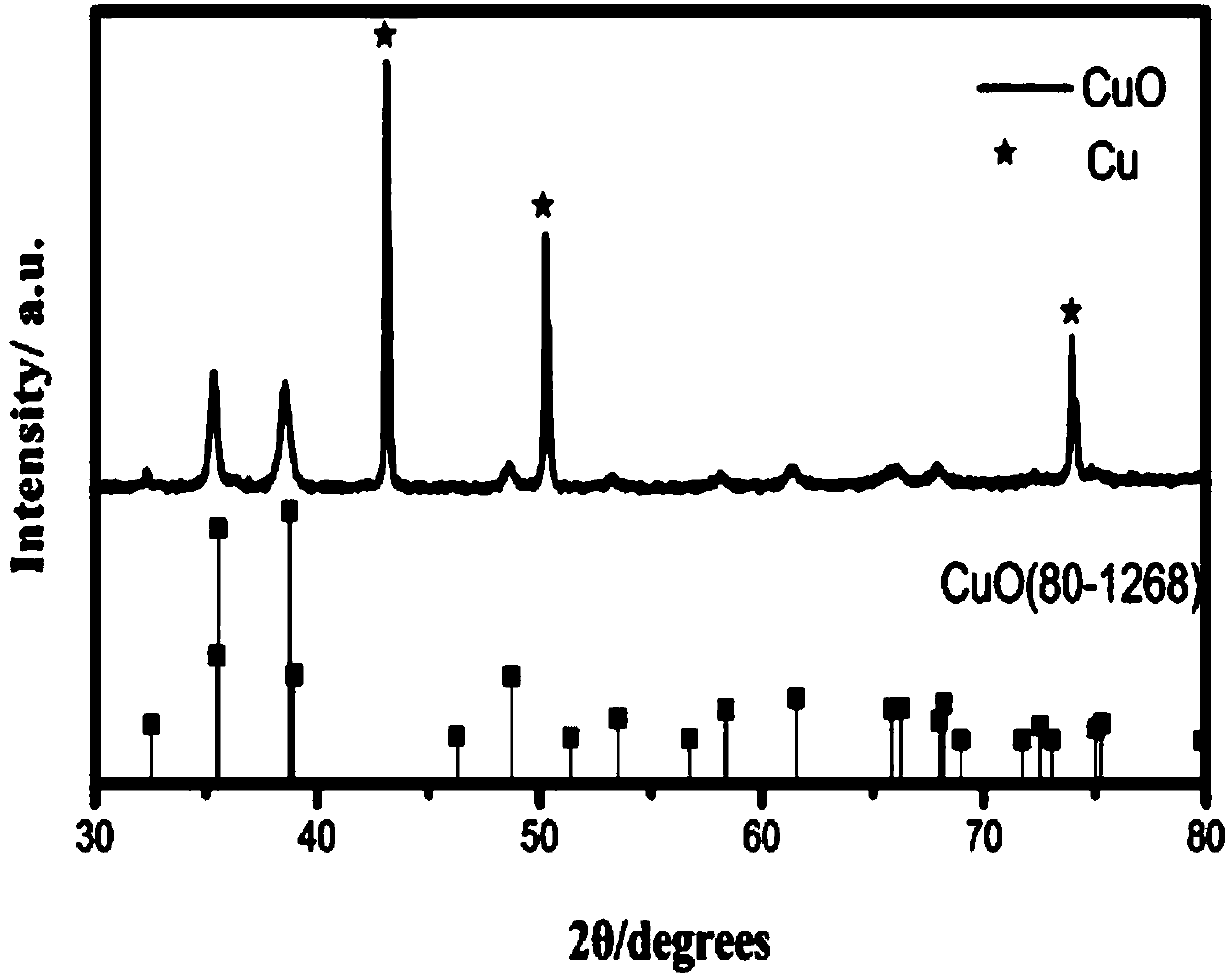
[0036] Such as Figure 1-Figure 8 As shown, the X-ray powder diffraction test results show that:
[0037] The diffractograms of a series of Co0.2, Co0.5, Co, 1.0 and Co1.5 catalysts of the present invention an...
Embodiment 3
[0040] In a conventional H-type electrolytic cell reaction system, the three-dimensional six-pointed star-shaped Co 3 o 4 CuO and nanosheet stacked flower-like CuO were used as anode and cathode materials respectively, and a 0.1 mol / L sodium sulfate solution (60 ml in volume) was added to the anode compartment, and a 0.1 mol / L potassium bicarbonate solution (60 ml in volume) was added to the cathode compartment. , and then add 0.5 g / L persulfate and 10 mg / L p-nitrophenol solution to the anolyte, and electrify to carry out electrolysis and advanced oxidation coupling reaction.
[0041] When the applied bias is -0.8V vs Ag / AgCl, Co 3 o 4 The removal rate of p-nitrophenol at the electric anode can reach more than 98%, and the CuO electric cathode can simultaneously reduce carbon dioxide to methanol and ethanol with yields of 49.145 and 20.475 micromoles / liter / hour, respectively. The Co of the present invention can be confirmed by the full-band degradation data 0.5 Catalyst ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com