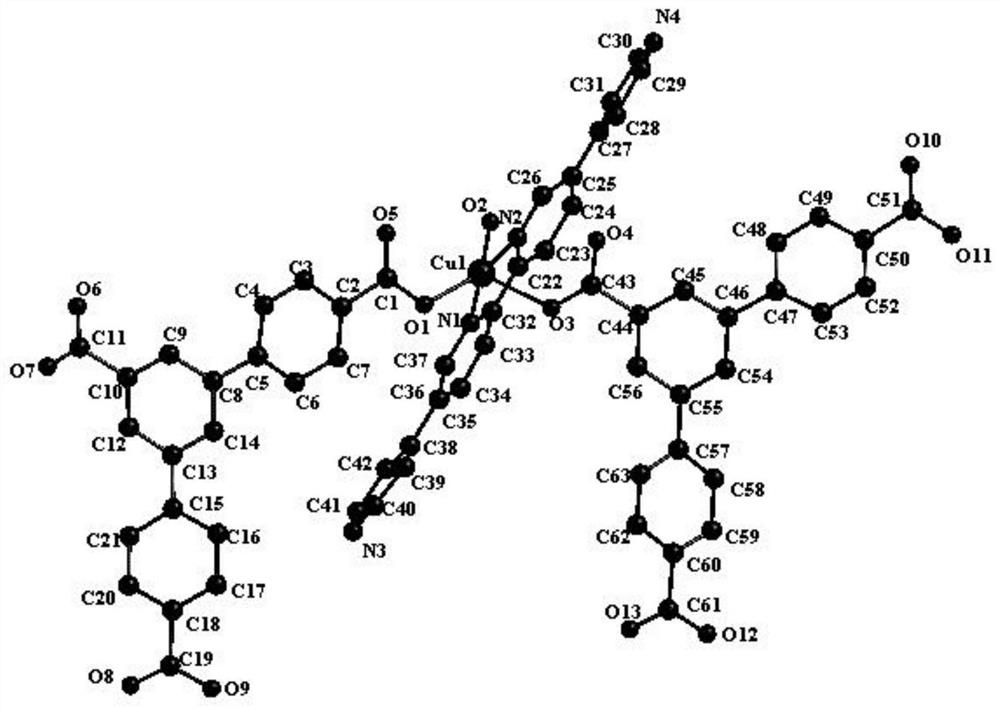
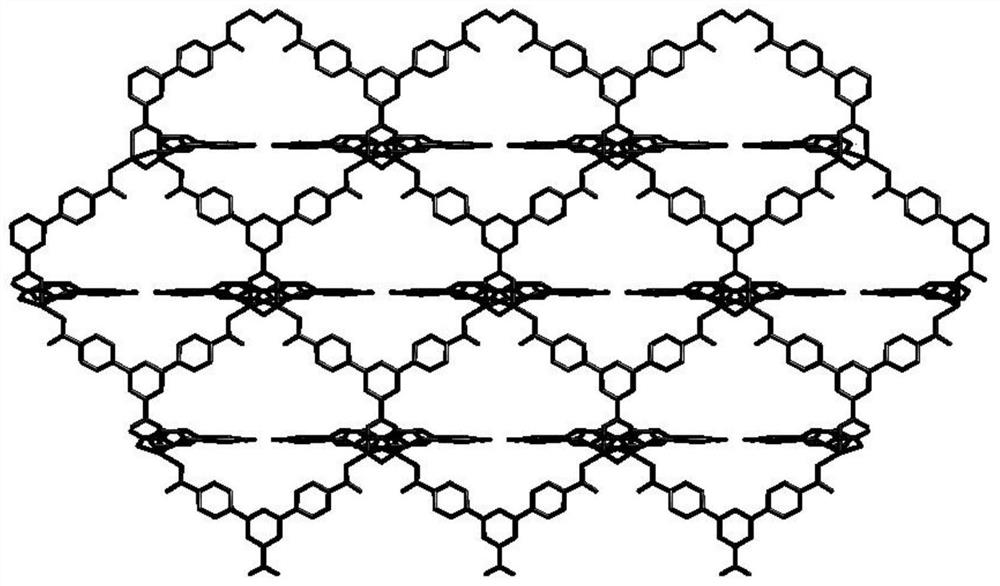
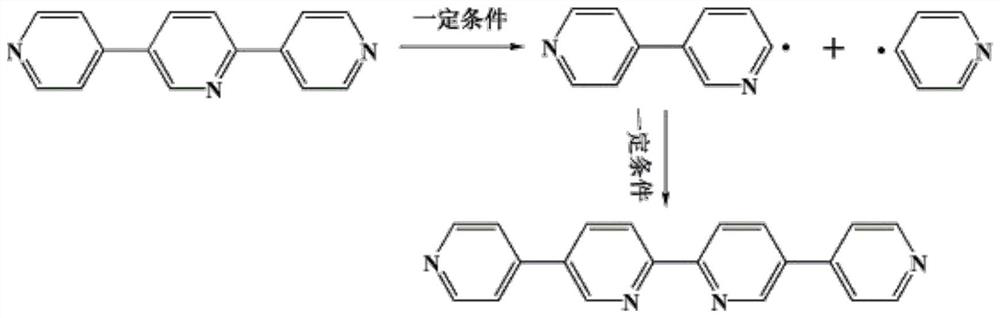
Synthesis of a copper compound and its application in photodegradation of organic dye methyl violet
A copper compound and reaction technology, applied in the field of copper compounds, can solve the problems of unreported research on synthetic compounds, harsh reaction conditions, difficulties, etc., and achieve the effects of low production cost, good thermal stability and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthesis of the copper compound that the present invention proposes, method step is as follows:
[0033] S1: A mixture of 0.1mmol of 2,5-bis(4-pyridyl)pyridine ligand and 0.08mmol of 1,3,5-tri(4-carboxyphenyl)benzene ligand, 0.15mmol of CuSO 4 Add to a 10ml reaction kettle, then add 1.0ml of N,N-dimethylformamide, 0.8ml of acetonitrile and 0.8ml of deionized water, and adjust the pH of the solution to 7.5 with NaOH;
[0034] S2: Heat the mixed solution adjusted in S1 to 85° C. for 5 days, and after the reaction is completed, lower the temperature to room temperature at a rate of 4° C. / h to obtain a copper compound.
Embodiment 2
[0036] The synthesis of the copper compound that the present invention proposes, method step is as follows:
[0037] S1: A mixture of 0.1mmol of 2,5-bis(4-pyridyl)pyridine ligand and 0.12mmol of 1,3,5-tri(4-carboxyphenyl)benzene ligand, 0.25mmol of CuSO 4 Add 10ml of reaction kettle, then add 2.0ml of N,N-dimethylformamide, 1.2ml of acetonitrile and 1.2ml of deionized water, and adjust the pH of the solution to 7.5 with NaOH;
[0038] S2: Heating the mixed solution adjusted in S1 to 95° C. for 7 days, and then lowering the temperature to room temperature at a rate of 6° C. / h after the reaction to obtain a copper compound.
Embodiment 3
[0040] The synthesis of the copper compound that the present invention proposes, method step is as follows:
[0041] S1: A mixture of 0.1mmol of 2,5-bis(4-pyridyl)pyridine ligand and 0.1mmol of 1,3,5-tri(4-carboxyphenyl)benzene ligand, 0.2mmol of CuSO 4 Add 10ml of reaction kettle, then add 1.5ml of N,N-dimethylformamide, 1.0ml of acetonitrile and 1.0ml of deionized water, and adjust the pH of the solution to 7.5 with NaOH;
[0042] S2: Heat the mixed solution adjusted in S1 to 90° C. for 6 days, and after the reaction is completed, lower the temperature to room temperature at a rate of 5° C. / h to obtain a copper compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com