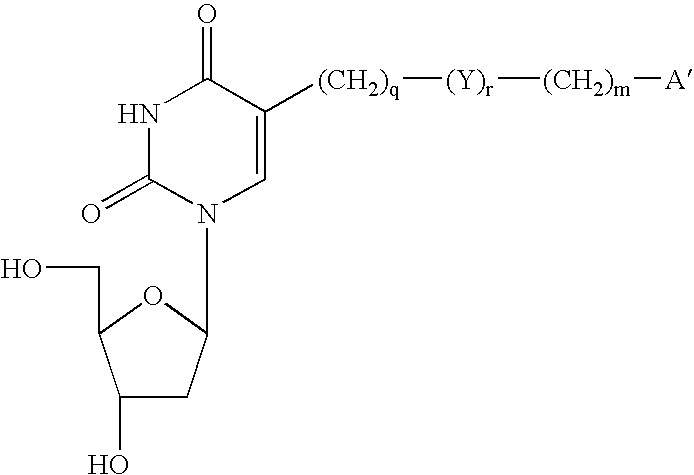
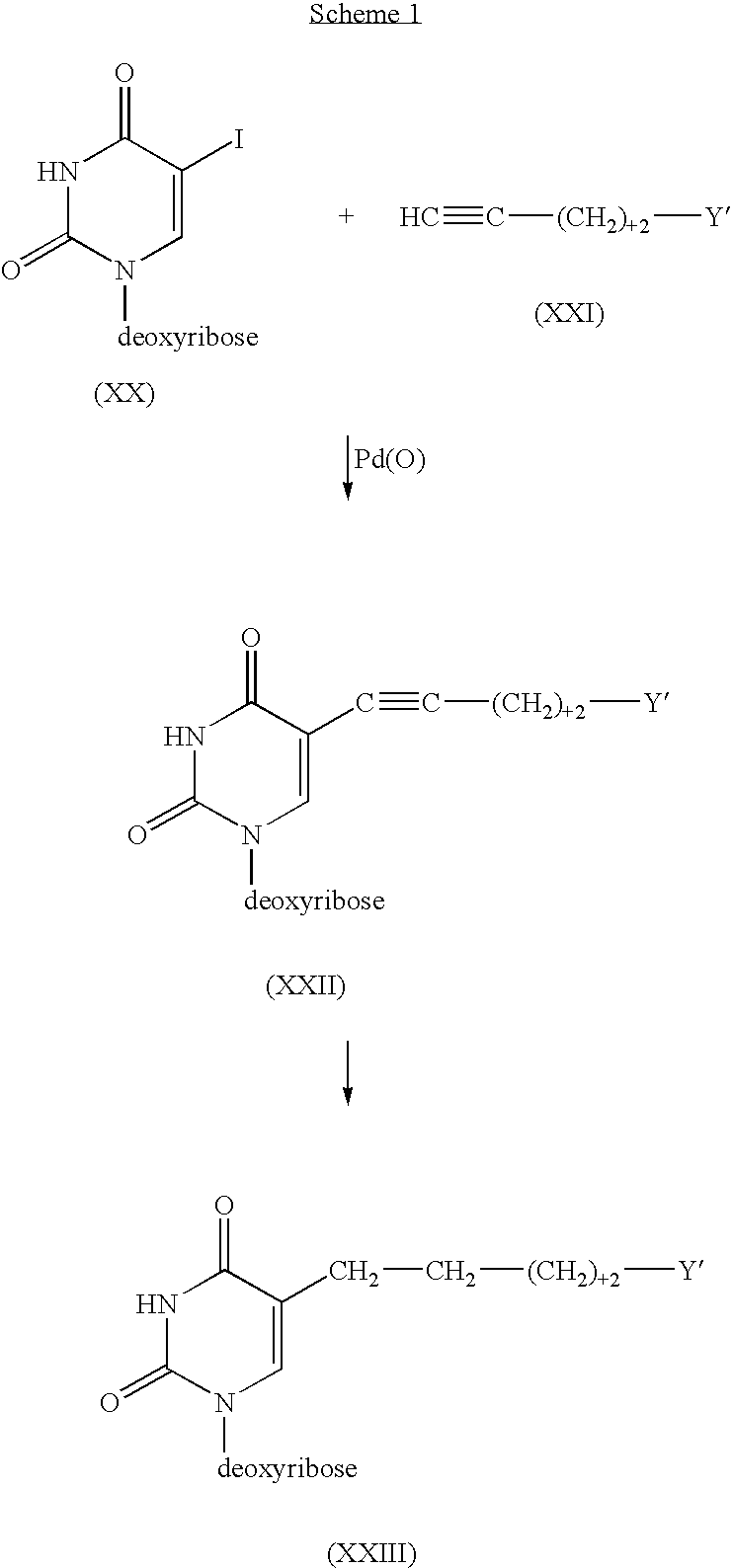
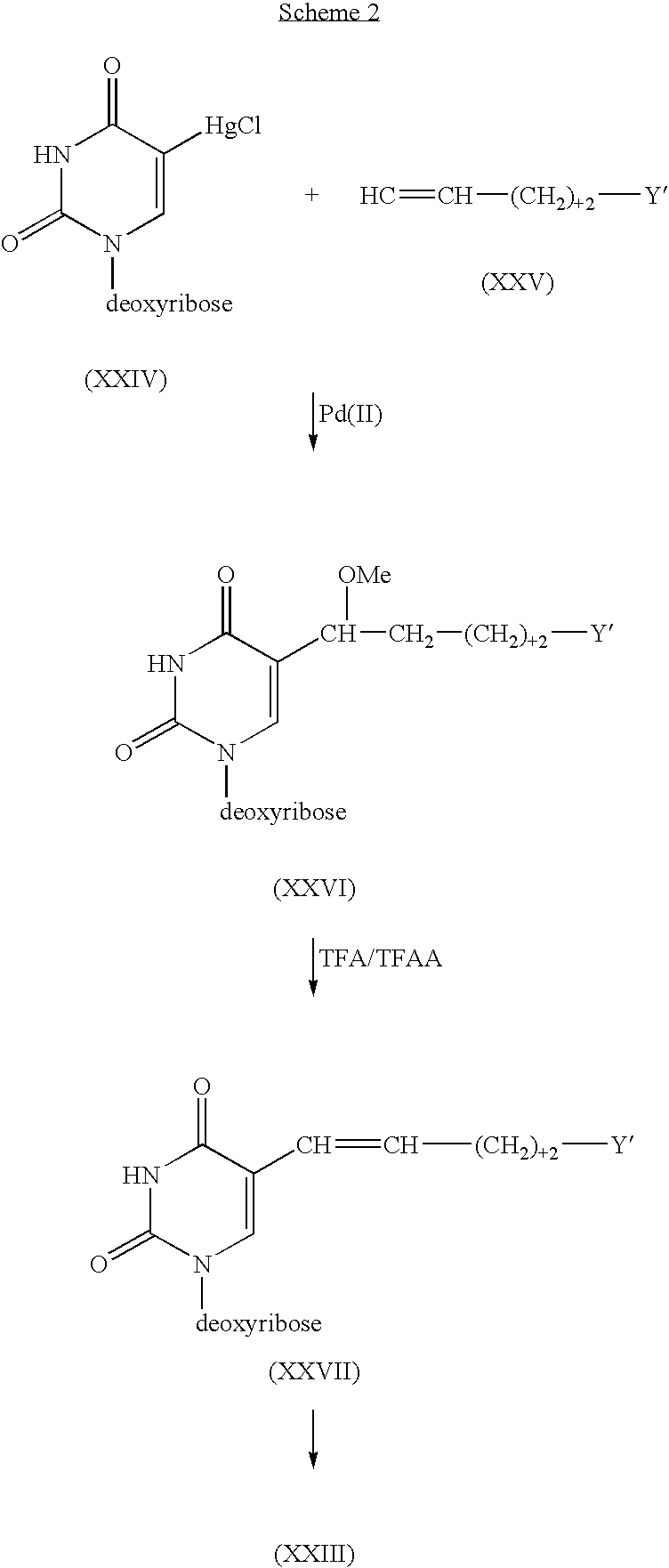
Cross-linking oligonucleotides
a cross-linking oligonucleotide and oligonucleotide technology, applied in the field of nucleoside cross-linking agents, can solve the problems of host toxicity, potential problems that must be circumvented,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
6-(Tritylamino)caproic Acid
6-Aminocaproic acid (26 g, 0.2 mole) was dissolved in dichloromethane (200 mL) by the addition of triethylamine (100 mL). Trityl chloride (120 g, 0.45 mol) was added and the solution stirred for 36 hr. The resulting solution was extracted with 1N HCl and the organic layer evaporated to dryness. The residue was suspended in 2-propanol / 1N NaOH (300 mL / 100 mL) and refluxed for 3 hr. The solution was evaporated to a thick syrup and added to dichloromethane (500 mL). Water was added and acidified. The phases were separated, and the organic layer dried over sodium sulfate and evaporated to dryness. The residue was suspended in hot 2-propanol, cooled, and filtered to give 43.5 g (58%) of 6-(trityl-amino)caproic acid, useful as an intermediate compound.
example 2
5-(Tritylamino) pentylhydroxymethylenemalononitrile
To a dichloromethane solution of 6-(tritylamino)-caproic acid (20.0 g, 53 mmole) and triethylamine (20 mL) in an ice bath was added dropwise over 30 min isobutylchloroformate (8.3 mL, 64 mmole). After the mixture was stirred for 2 hr in an ice bath, freshly distilled malononitrile (4.2 g, 64 mmole) was added all at once. The solution was stirred for 2 hr in an ice bath and for 2 hr at RT. The dichloromethane solution was washed with ice cold 2N HCl (300 mL) and the biphasic mixture was filtered to remove product that precipitated (13.2 g). The phases were separated and the organic layer dried and evaporated to a thick syrup. The syrup was covered with dichloromethane and on standing deposited fine crystals of product. The crystals were filtered and dried to give 6.3 g for a total yield of 19.5 g (87%) of the product, which is useful as an intermediate.
example 3
5-(Tritylamino) pentylmethoxymethylenemalononitrile
A suspension of the malononitrile of Example 2 (13 g, 31 mmole) in ether / dichloromethane (900 mL / 100 mL), cooled in an ice bath, was treated with a freshly prepared ethereal solution of diazomethane (from 50 mmole of Diazald.RTM. (Aldrich Chemical Company)). The solution was stirred for 6 hr and then neutralized with acetic acid (10 mL). The solution was evaporated to dryness and the residue chromatographed on silica gel using dichloromethane / acetone (4 / 1) as the eluent. Fractions containing product were pooled and evaporated to a syrup. The syrup was triturated with dichloromethane to induce crystallization. The crystals were filtered and dried to give 8.3 g (61%) of chromatographically pure product, useful as an intermediate compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com