Method for preparing enamine compound by catalyzing phenylacetylene and utilizing hydroamination reaction
A technology of phenylacetylene and hydroamination, which is applied in the field of catalytic chemistry, can solve the problems of low tolerance of functional groups, limited types of amino reagents, and high requirements for reaction conditions, and achieves easy availability of raw materials, high physical and chemical stability and Effects of thermal stability and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
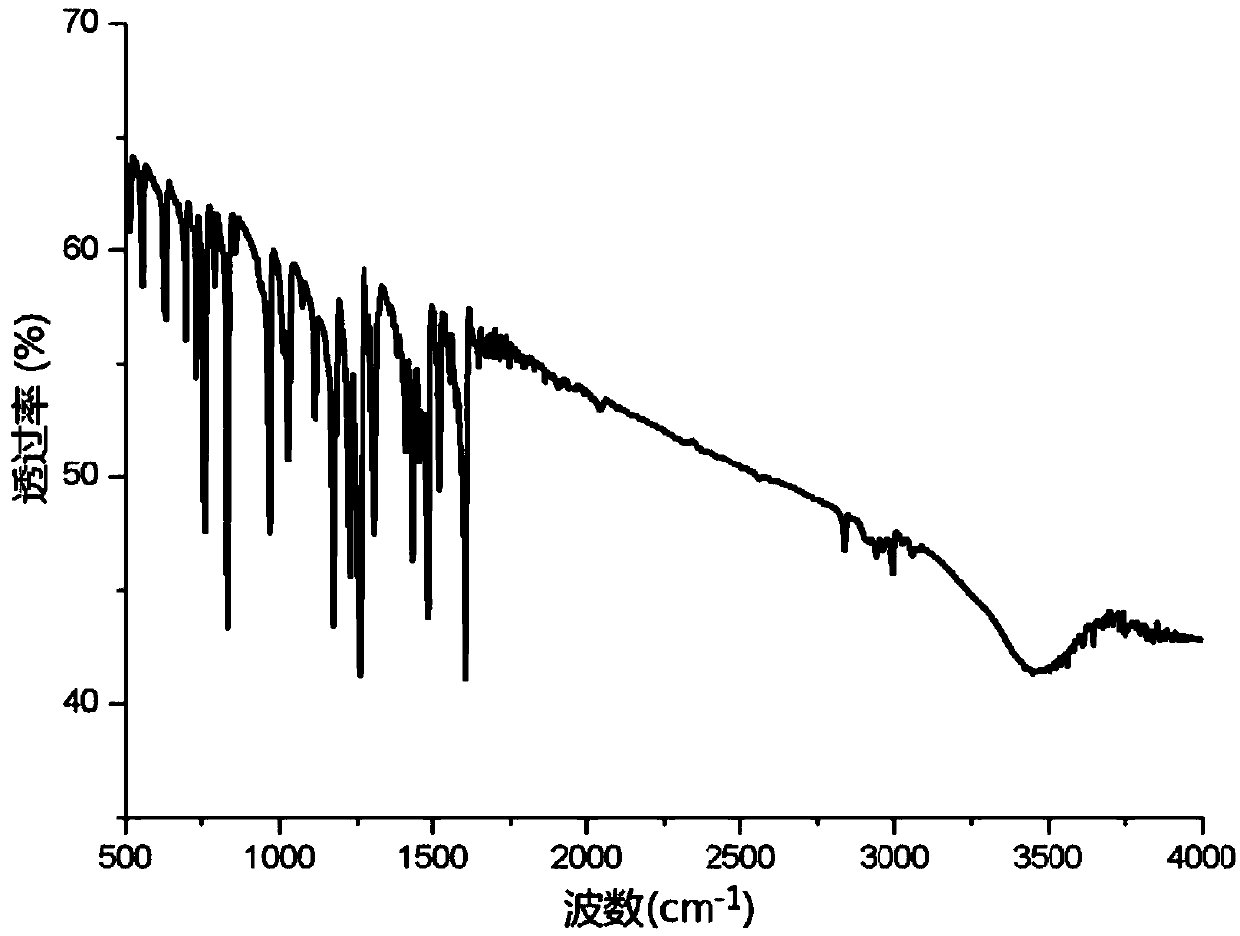
[0028] Example 1: Divalent nickel imine complexes catalyze the hydroamination of phenylacetylene to prepare enamines
[0029]
[0030] Catalyst [CH 2 (C 5 h 4 N) 2 ] Ni=NPh (3.2mg, 0.00001mol), phenylacetylene (1.02g, 0.01mol) and methylphenylamine (1.02g, 0.01mol) and 5mL toluene were added to the reaction tube, and phenylacetylene and methylphenylamine As a raw material, under the action of a divalent nickel imine complex catalyst, hydroamination reaction occurs in the solvent toluene, the reaction temperature is 60°C, and the reaction time is 8h. After the end, the system is extracted with ethyl acetate for 3 times, and the liquid is separated , the organic phase was dried with anhydrous sodium sulfate, then filtered and concentrated, and the concentrated reaction solution was separated by silica gel column chromatography (eluent: dichloromethane: ethyl acetate = 5: 1) to obtain the corresponding enamine compound. The resulting product was subjected to LC-MS to obtai...
Embodiment 2
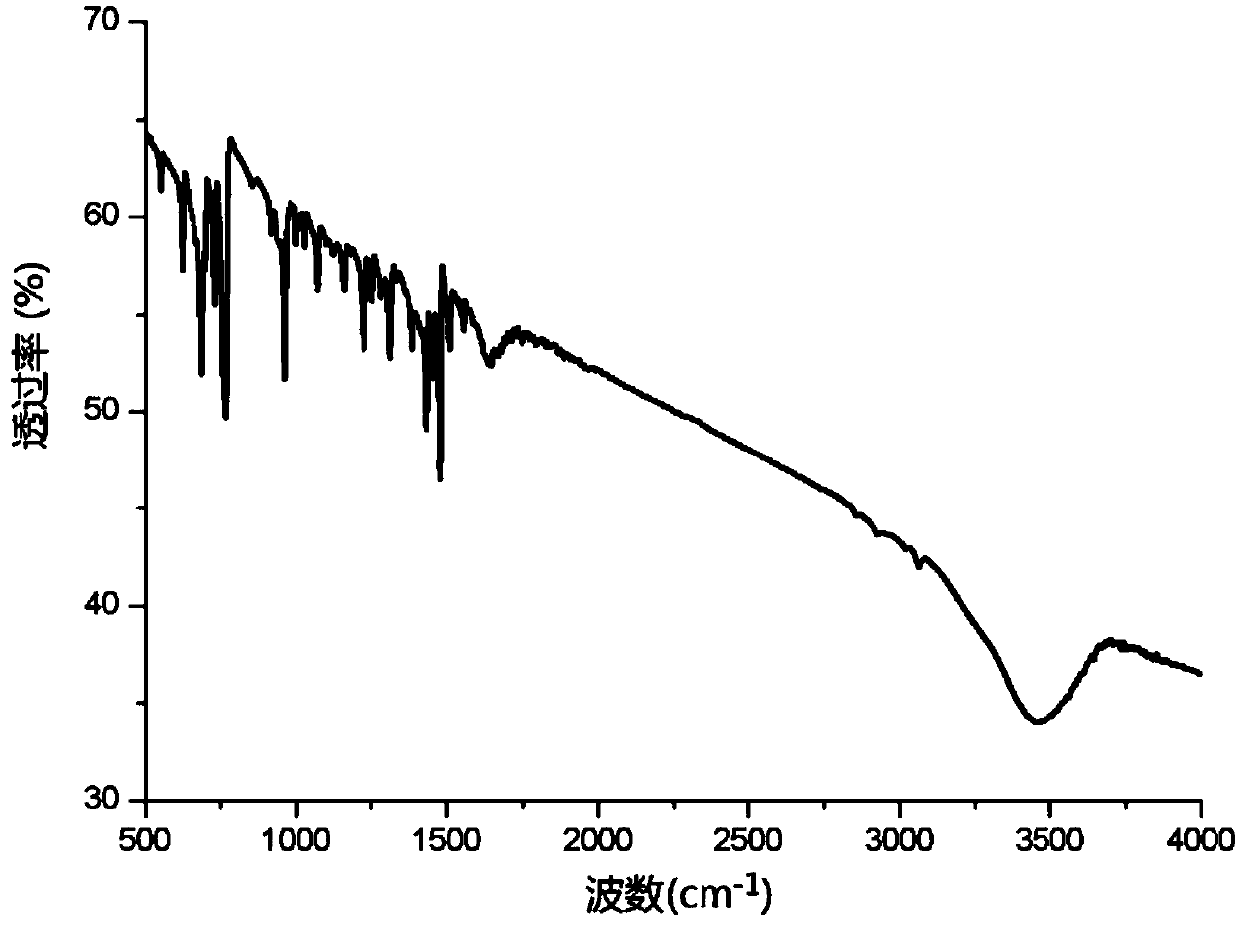
[0031] Example 2: Divalent nickel imine complexes catalyze the hydroamination of phenylacetylene to prepare enamines
[0032]
[0033] The catalyst [(CH 3 )CH(C 5 h 4 N) 2 ] Ni=NPh (3.3mg, 0.00001mol), phenylacetylene (1.22g, 0.012mol) and methylethylamine (1.02g, 0.01mol) and 8mL toluene were added to the reaction tube, and phenylacetylene and methylethylamine As a raw material, under the action of a divalent nickel imine complex catalyst, hydroamination reaction occurs in the solvent toluene, the reaction temperature is 75°C, and the reaction time is 6h. After the end, the system is extracted with ethyl acetate for 3 times, and the liquid is separated. , the organic phase was dried with anhydrous sodium sulfate, then filtered and concentrated, and the concentrated reaction solution was separated by silica gel column chromatography (eluent: dichloromethane: ethyl acetate = 5: 1) to obtain the corresponding enamine compound. The resulting product was subjected to LC-MS ...
Embodiment 3
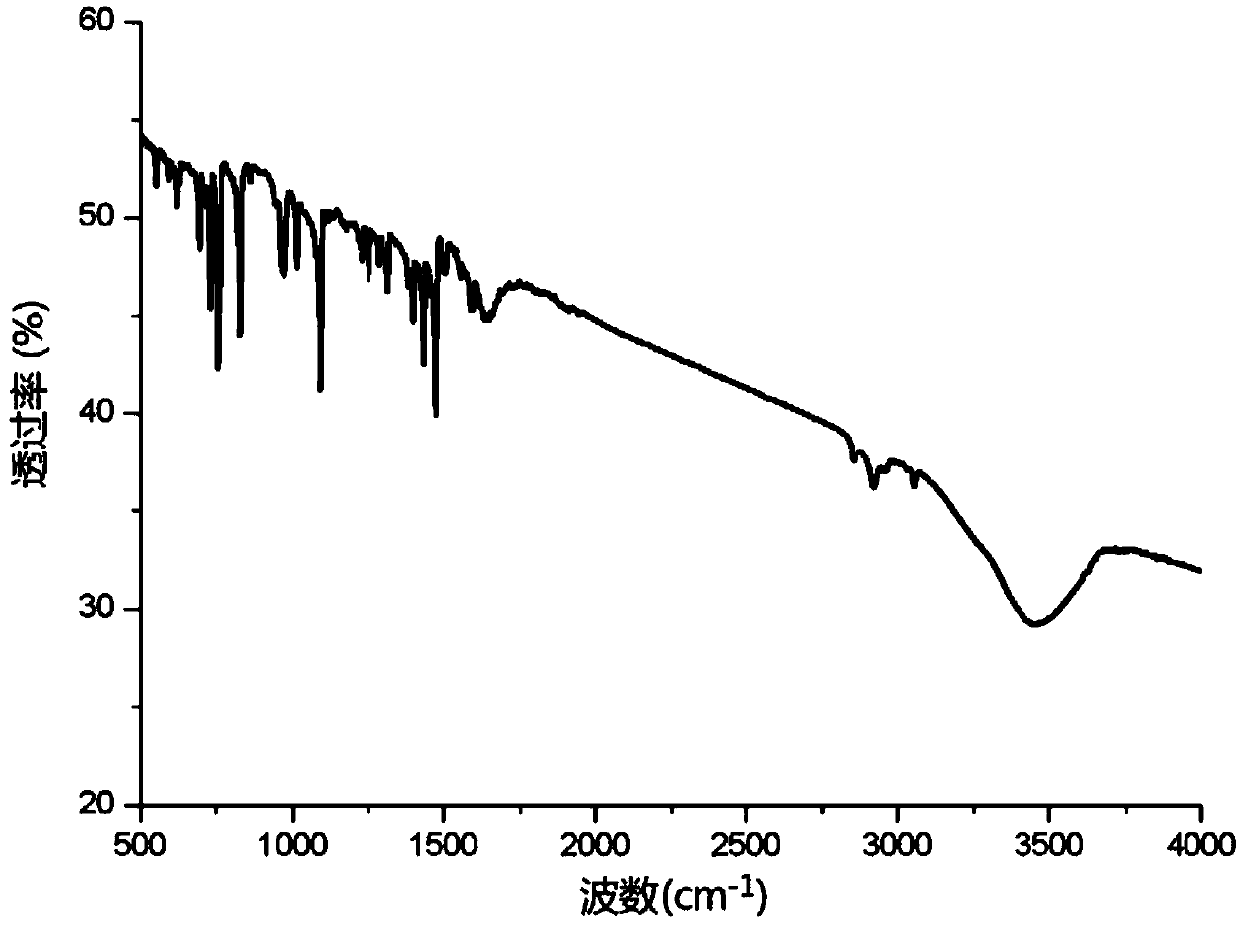
[0034] Example 3: Divalent nickel imine complexes catalyze the hydroamination of phenylacetylene to prepare enamines
[0035]
[0036] The catalyst [PhCH(C 5 h 4 N) 2 ] Ni=NPh (4.0mg, 0.00001mol), phenylacetylene (1.53g, 0.015mol) and methyl isopropylamine (1.02g, 0.01mol) and 8mL toluene were added to the reaction tube, and phenylacetylene and methyl isopropyl Base amine is used as raw material, under the action of divalent nickel imine complex catalyst, hydroamination reaction occurs in solvent toluene, the reaction temperature is 100°C, and the reaction time is 12h. After the end, the system is extracted with ethyl acetate for 3 times. Separate the liquid, dry the organic phase with anhydrous sodium sulfate, filter and concentrate, and separate the concentrated reaction solution through silica gel column chromatography (eluent: dichloromethane:ethyl acetate=5:1) to obtain the corresponding enamine compound , LC-MS was performed on the resulting product to obtain 1.522...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com