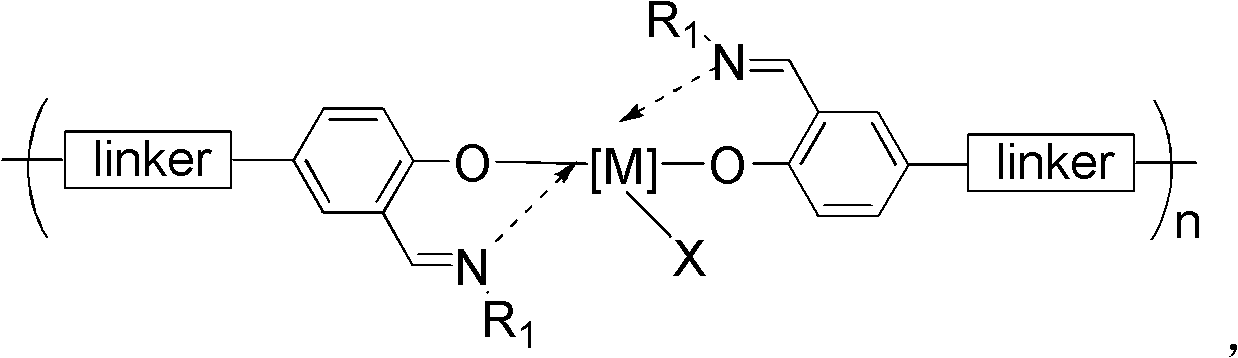
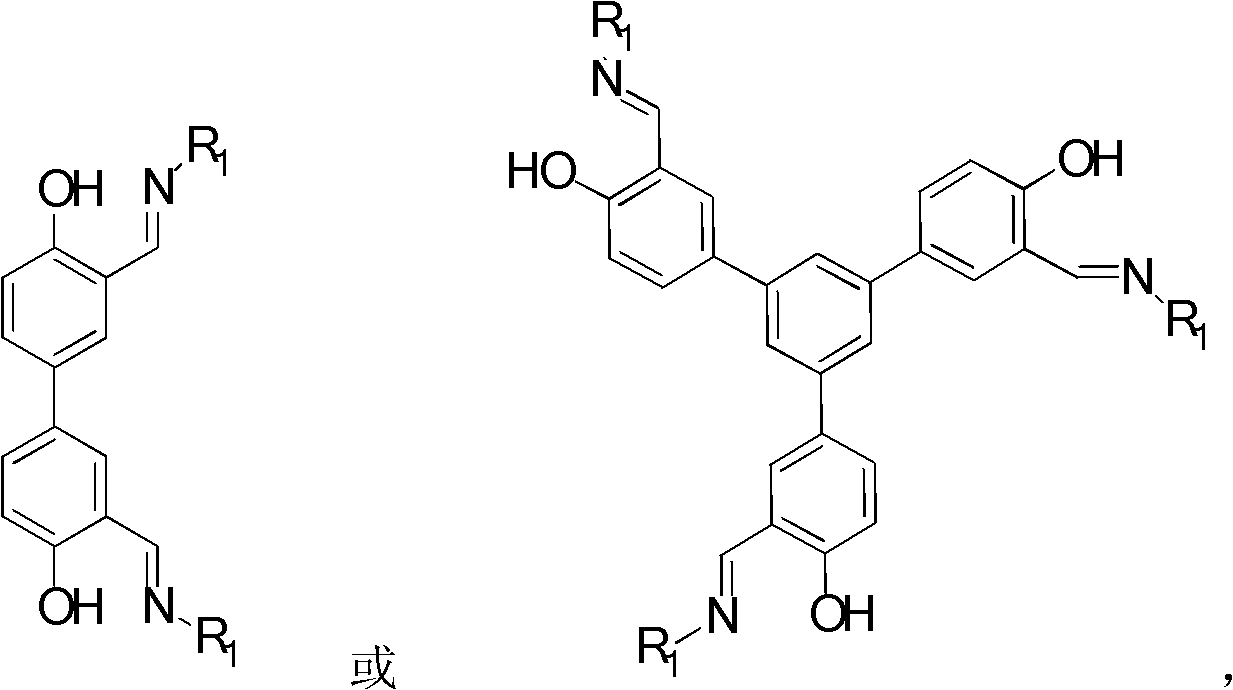
Schiff-base ligand-based rare-earth metal complex, preparation method and applications
A technology of rare earth metals and Schiff bases, applied in the direction of organic compound/hydride/coordination complex catalysts, compounds containing elements of group 3/13 of the periodic table, chemical instruments and methods, etc. Problems such as recycling results, low ee value, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078]
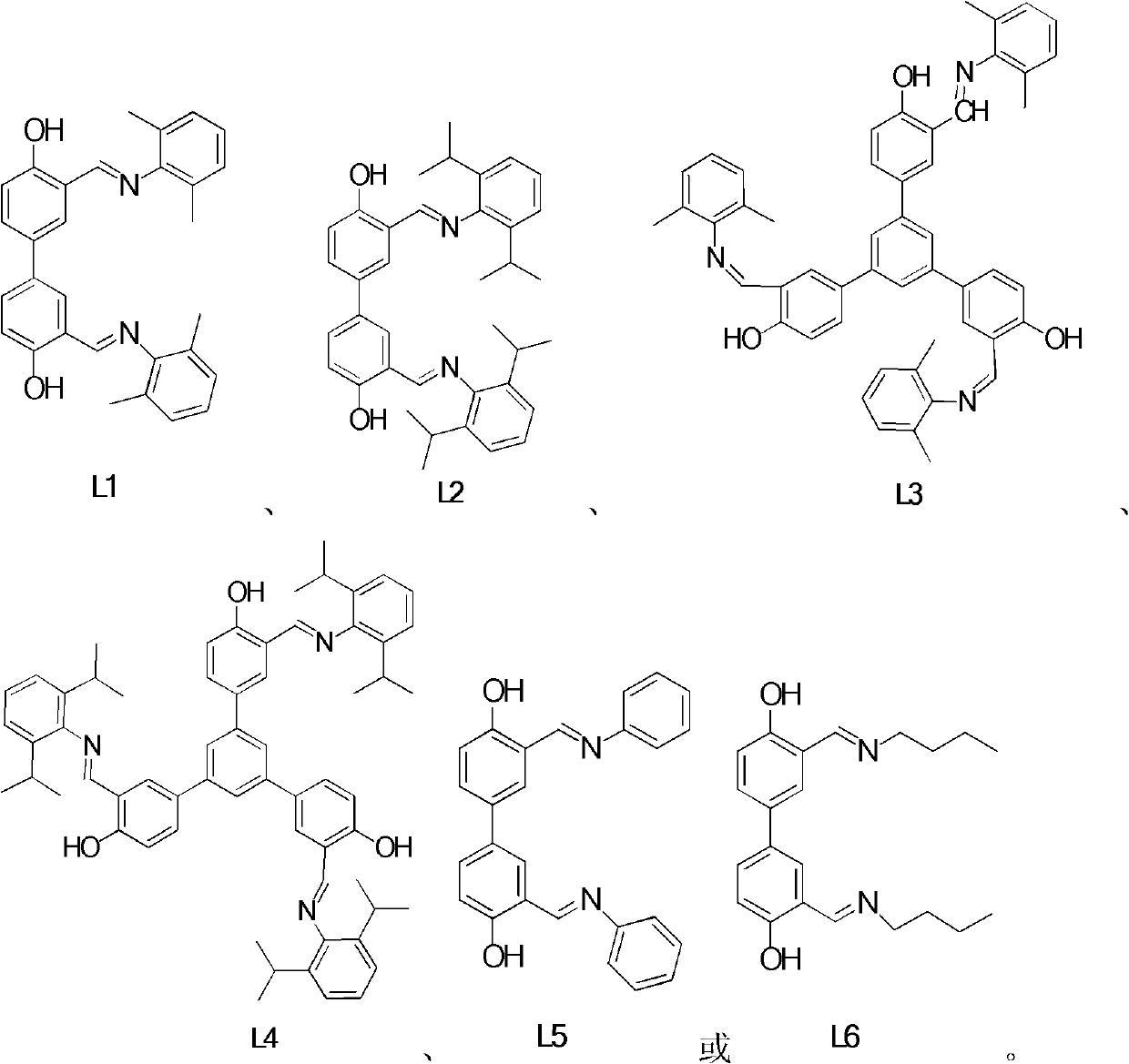
[0079] In a 50mL single-necked bottle, add 4,4'-dihydroxy-3,3'-dialdehyde biphenyl (127g, 52mmol), 2,6-dimethylaniline (0.23mL, 1.05mmol) and 10mL absolute ethanol , heated to reflux for 2 hours. The solvent was removed, and the residual solid was recrystallized from absolute ethanol to obtain 3,3'-bis-(2,6-dimethylbenimine)methyl)biphenyl-4,4'-diol L1 (140mg, 60 % yield (yield)). 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 13.14 (s, 2H, OH)), 8.42 (s, 2H, N=CH), 7.58 (dd, J=2.4, 3.6Hz, 2H, ArH), 7.52 (d, J=2.4 Hz, 2H, ArH), 7.11-7.15(m, 6H, ArH), 7.01-7.06(m, 2H, ArH), 2.22(s, 24H, CH 3 ); 13 C NMR (100MHz, CDCl 3 ): δ (ppm) 116.4, 110.4, 97.9, 81.0, 80.8, 79.6, 77.8, 77.7, 77.5, 74.5, 68.5, 67.5. HRMS (MALDI): C 30 h 29 N 2 o 2 (M + ) calculated value: 449.2223; measured value: 449.2224.
Embodiment 2
[0081]
[0082] In a 50mL single-necked bottle, add 4,4'-dihydroxy-3,3'-dialdehyde biphenyl (127mg, 0.52mmol), 2,6-diisopropylaniline (0.26mL, 1.05mmol) and 10mL without water and ethanol, heated to reflux for 2 hours. The solvent was removed, and the residual solid was recrystallized from absolute ethanol to obtain 3,3'-bis-(2,6-diisopropylphenylimine)methyl)biphenyl-4,4'-diol L2 (150mg, 63% yield). 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 13.15 (s, 2H, OH)), 8.39 (s, 2H, CH=N), 7.64 (dd, J=2, 2Hz, 2H, ArH), 7.53 (d, J=2Hz, 2H, ArH), 7.20(s, 6H, ArH), 7.16(d, J=8.8Hz, 2H, ArH), 2.98-3.05(m, 4H, CH(CH 3 ) 2 ), 1.19(d, J=7.2Hz, 24H, CH(CH 3 ) 2 ); 13C NMR (100MHz, CDCl 3 ): δ (ppm) 166.7, 160.6, 146.2, 138.8, 131.7, 131.6, 130.2, 125.7, 123.4, 118.9, 28.3, 23.7. HRMS (MALDI): C 38 h 45 N 2 o 2 (M + ) calculated value: 561.3482; measured value: 561.3476.
Embodiment 3
[0084]
[0085] In a 50mL single-necked bottle, add 1,3,5-tris(4-hydroxy-5-formylphenyl)benzene (1g, 2.3mmol), 2,6-dimethylaniline (1.5mL, 6.8mmol), without A mixed solvent of water, ethanol and toluene (v / v=2 / 1), heated under reflux for 2 hours to complete the reaction. The solvent was removed, and the residual solid was recrystallized from absolute ethanol to obtain 1,3,5-tris-(4-hydroxy-5-(2,6-dimethylbenimine)methyl)benzene L3 (1.4g, 65 %yield). 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 13.22 (s, 3H, OH), 8.45 (s, 3H, CH=N), 7.76 (dd, J=2.0, 2.4Hz, 3H, ArH), 7.69 (s, 3H, ArH) , 7.65 (d, J=2.4Hz, 3H, ArH), 7.18 (m, 3H, ArH), 7.11 (m, 6H, ArH), 7.04 (m, 3H, ArH), 2.23 (s, 18H, CH 3 ); 13 C NMR (100MHz, CDCl 3 ): δ (ppm) 166.8, 161.2, 148.1, 141.7, 132.3, 132.2, 130.8, 128.5, 128.4, 125.2, 123.9, 119.1, 118.1, 18.6. HRMS (MALDI): C 51 h 46 N 3 o 3 (M + ) calculated value: 748.3547; measured value: 748.3533.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com