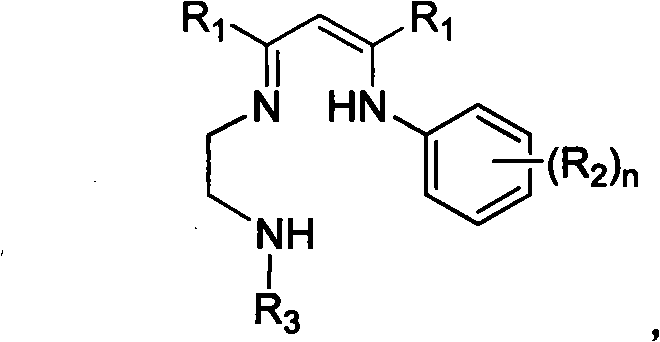
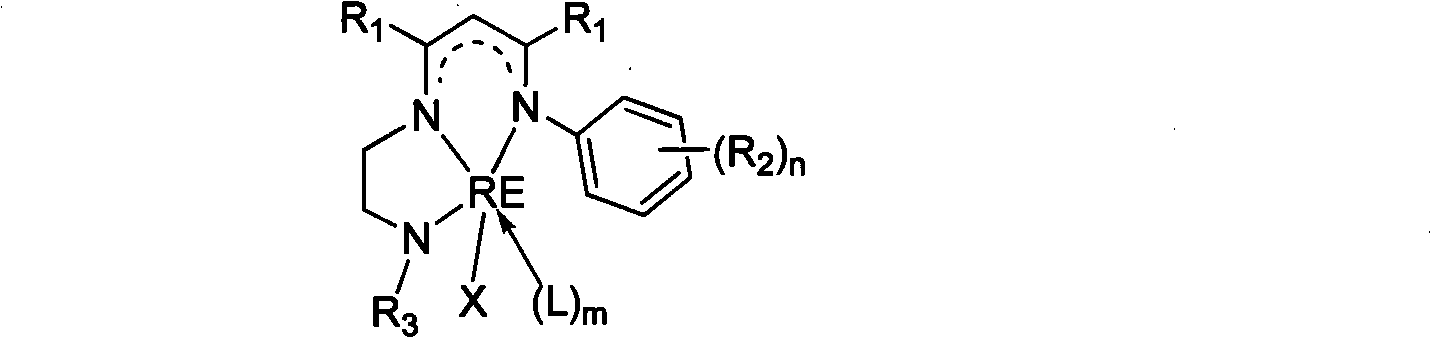
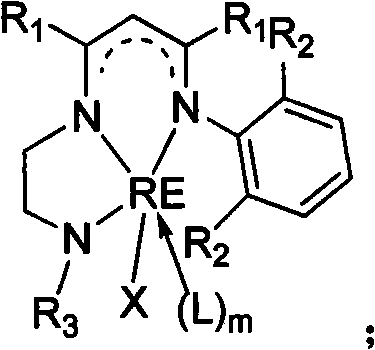
Novel three-tooth nitrogen ligand and rare earth metal complex
A technology of rare earth complexes and rare earth metals, which can be used in the preparation of organic compound/hydride/coordination complex catalysts, organic silicon compounds, imino compounds, etc., which can solve problems such as environmental pollution and materials that do not have biodegradable properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In a 250mL three-necked flask, add 2-(2,6-diisopropylanilino)-2-penten-4-one (5.5g, 21.5mmol), N-tert-butyl-(1,2-ethanedi amine) (2.5 g, 21.5 mmol), a catalytic amount of p-toluenesulfonic acid, and 100 mL of toluene. Under the protection of nitrogen, the water was refluxed for 24 hours. Finally, 13.4 g (136° C. / 5 Pa) of the tridentate nitrogen ligand L was obtained as a light yellow oily product, with a yield of 45%. by 1 HNMR, 13 C NMR and mass spectrometry characterized the structure of the ligands. 1 H NMR (300MHz, C 6 D. 6 , 25°C): δ (ppm) 11.03 (br, 1H, MeC (NH) CH), 7.06-7.18 (m, 3H, ArH), 4.67 (s, 1H, MeC (N) CH), 3.14 (sp, 3 J HH = 6.6Hz, 2H, ArCHMe 2 ), 2.97 (q, 2H, NCH 2 ), 2.45(t, 3 J HH = 6.3Hz, 2H, NCH 2 ), 1.68(s, 3H, MeC), 1.64(s, 3H, MeC), 1.24(d, 3 J HH = 6.9Hz, 6H, ArCHMe 2 ), 1.20(d, 3 J HH = 6.9Hz, 6H, ArCHMe 2 ), 0.88(s, 9H, NCMe 3 ), 0.45 (br, 1H, t Bu-NH). 1 H NMR (300MHz, CDCl 3 , 25°C): δ (ppm) 10.83 (br, 1H, MeC (NH) CH),...
Embodiment 2
[0044] In a 250mL three-necked flask, add 2-(2,6-diisopropylanilino)-2-penten-4-one (14.6g, 56.3mmol), N-2,6-dimethylphenyl- (1,2-Ethylenediamine) (9.5 g, 57.8 mmol), catalytic amount of p-toluenesulfonic acid and 100 mL of toluene. Under the protection of nitrogen, the water was refluxed for 24 hours. After the reaction was complete, the solvent was removed in vacuo to obtain a brown viscous substance. The tridentate nitrogen ligand L212g was obtained as a white solid by recrystallization from methanol with a yield of 52%. by 1 H NMR, 13 C NMR and mass spectrometry characterized the structure of the ligands. 1 H NMR (300MHz, C 6 D. 6 , 25°C): δ (ppm) 11.18 (br, 1H, MeC (NN) CH), 7.13-7.22 (m, 3H, ArH), 6.87-6.96 (m, 3H, ArH), 4.71 (s, 1H, MeC(NH)CH), 3.17(sp, 3 J HH =7.2Hz, 2H, ArCHMe 2 ), 3.15 (br, 1H, NH), 2.92 (br, 2H, NCH 2 ), 2.86 (br, 2H, NCH 2 ), 2.14(s, 6H, ArMe), 1.67(s, 3H, MeC), 1.58(s, 3H, MeC), 1.22(d, 3 J HH = 6.9Hz, 6H, ArCHMe 2 ), 1.21(d, 3 J ...
Embodiment 3
[0046]In a 250mL three-necked flask, add 2-(2,6-diisopropylanilino)-2-penten-4-one (7.06g, 27.2mmol), N-2,6-diisopropylphenyl -(1,2-Ethylenediamine) (6 g, 27.2 mmol), catalytic amount of p-toluenesulfonic acid and 100 mL of toluene. Under the protection of nitrogen, the water was refluxed for 24 hours. After the reaction was complete, the solvent was removed in vacuo to obtain a brown viscous substance. Methanol recrystallization gave 35.5 g of tridentate nitrogen ligand L as a white solid, with a yield of 44%. by 1 HNMR, 13 CNMR and mass spectrometry characterized the structure of the ligand. 1 HNMR (300MHz, C 6 D. 6 , 25°C): δ (ppm) 11.29 (br, s, 1H, MeC(NH)CH), 7.24-7.25 (br, m, 6H, ArH), 4.80 (s, 1H, MeC(NH)CH), 3.36 (sp, 3 J HH =7.2Hz, 2H, ArCHMe 2 ), 3.23 (sp, 3 J HH = 6.9Hz, 2H, ArCHMe 2 ), 3.15 (br, 2H, NCH 2 ), 2.98 (br, 2H, NCH 2 ), 1.75(s, 3H, MeC), 1.74(s, 3H, MeC), 1.29(d, 3 J HH = 7.2Hz, 12H, ArCHMe 2 ), 1.25(d, 3 J HH = 6.9Hz, 12H, ArCHMe 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com