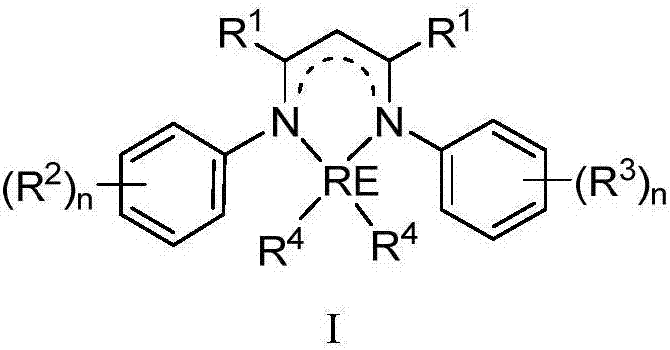
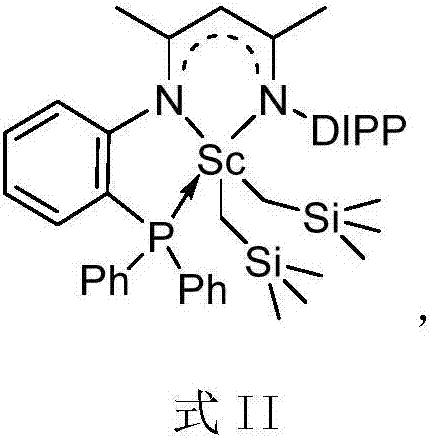
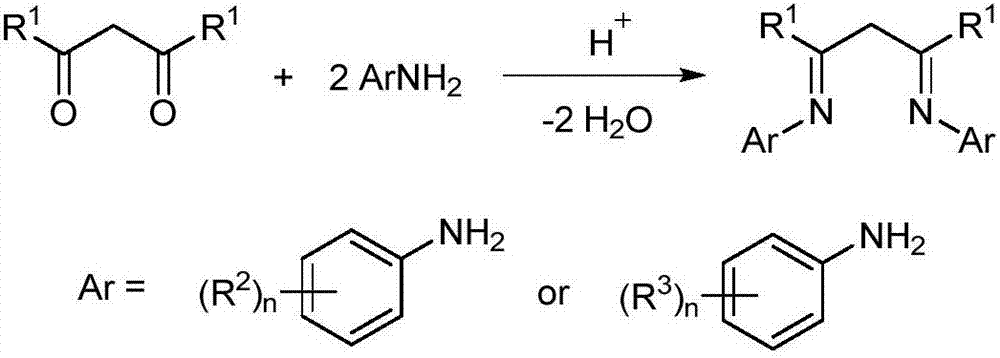
Rare earth complex based on diimine ligands and application of rare earth complex
A diimine ligand, rare earth metal technology, applied in the field of chemistry, can solve the problem of inability to catalyze tertiary aromatic amines, etc., and achieve the effect of wide application range of substrates, excellent chemical selectivity and regioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]
[0043] Weigh L1 (35mg), [PhNHMe 2 ][B(C 6 f 5 ) 4 ] (38mg), dissolved in toluene, reacted at room temperature for 8 hours. After the reaction was complete, N,N-dimethylaniline (86 mg) and 1-hexene (40 mg) were added and heated at 120° C. for 10 hours. After the reaction was completed, the toluene was drained to obtain an oily residue, which was separated by column chromatography to obtain a light yellow oily liquid (89 mg, 91% yield).
[0044] HRMS (ESI) m / z calculated value: For C 14 h 23 N[M+H] + :206.1903; Measured value: 206.1909.
[0045] 1 H NMR (400MHz,C 6 D. 6 ,298K):δ=7.26(m,2H,m-Ph),6.78(m,1H,p-Ph),6.68(m,2H,o-Ph),3.00(dd, 2 J HH =14.5Hz, 3 J HH =6.6Hz,1H,CH 2 N),2.80(dd, 2 J HH =14.5Hz, 3 J HH =8.1Hz,1H,CH 2 N),2.63(s,3H,CH 3 N),1.78(m,1H,CHCH 3 ),1.23(m,1H,CHCH 2 ),1.23(m,1H,CH 2 ),1.23(m,2H,CH 2 CH 3 ),1.10(m,1H,CH 2 ),0.93(m,1H,CHCH 2 ),0.87(t, 3 J HH =6.9Hz,3H,CH 2 CH 3 ),0.77(d, 3 J HH =6.7Hz,3H,CHCH 3 ).
[0046]...
Embodiment 2
[0048]
[0049] Weigh L1 (35mg), [PhNHMe 2 ][B(C 6 f 5 ) 4 ] (38mg), dissolved in toluene, reacted at room temperature for 8 hours. After the reaction was complete, N,N-dimethylaniline (86 mg) and 1-decene (67 mg) were added and heated at 120° C. for 22 hours. After the reaction was completed, the toluene was drained to obtain an oily residue, which was separated by column chromatography to obtain a light yellow oily liquid (100 mg, 80% yield).
[0050] HRMS (ESI) m / z calculated value: For C 18 h 31 N[M+H] + :262.2529; Measured value: 262.2536.
[0051] 1 H NMR (400MHz,C 6 D. 6 ,298K): δ=7.26(m,2H,m-Ph),6.78(m,1H,p-Ph),6.69(m,2H,o-Ph),3.02(dd, 2 J HH =14.5Hz, 3 J HH =6.7Hz,1H,CH 2 N),2.82(dd, 2 J HH =14.5Hz, 3 J HH =8.0Hz,1H,CH 2 N),2.64(s,3H,CH 3 N),1.82(m,1H,CHCH 3 ),1.27(m,13H,CH 2 ),0.99(m,1H,CHCH 2 ),0.92(t, 3 J HH =6.8Hz,3H,CH 2 CH 3 ),0.79(d, 3 J HH =6.7Hz,3H,CHCH 3 ).
[0052] 13 C{ 1 H}NMR (101MHz,C 6 D. 6 ,298K): δ=150.2(i-Ph), 1...
Embodiment 3
[0054]
[0055] Weigh L1 (35mg), [PhNHMe 2 ][B(C 6 f 5 ) 4 ] (38mg), dissolved in toluene, reacted at room temperature for 8 hours. After the reaction was complete, N,N-dimethylaniline (86 mg) and allyltrimethylsilane (54 mg) were added and heated at 120° C. for 8 hours. After the reaction was completed, the toluene was drained to obtain an oily residue, which was separated by column chromatography to obtain a light yellow oily liquid (101 mg, 90% yield).
[0056] HRMS (ESI) m / z calculated value: For C 14 h 25 NSi[M+H] + :236.1829; Measured value: 236.1837.
[0057] 1 H NMR (400MHz,C 6 D. 6 ,298K):δ=7.26(m,2H,m-Ph),6.78(m,1H,p-Ph),6.70(m,2H,o-Ph),3.00(dd, 2 J HH =14.3Hz, 3 J HH =6.3Hz,1H,CH 2 N),2.80(dd, 2 J HH =14.3Hz, 3 J HH =8.5Hz,1H,CH 2 N),2.64(s,3H,CH 3 N),1.98(m,1H,CHCH 3 ),0.83(d, 3 J HH =6.6Hz,3H,CHCH 3 ),0.56(dd, 2 J HH =14.6Hz, 4 J HH =4.0Hz,1H,CH 2 SiMe 3 ),0.21(dd, 2 J HH =14.6Hz, 4 J HH =9.8Hz,1H,CH 2 SiMe 3 ),-0.01(s,9H,Si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com