Nickelous imine complex with nickel nitrogen double-bond structure and preparation and application of nickelous imine complex
A divalent nickel imine and complex technology, which is applied in the preparation of organic compounds, amino hydroxyl compounds, nickel organic compounds, etc., can solve the problems of few types of catalyzed substrates, harsh reaction conditions, and application limitations, etc., to achieve The effects of high physical and chemical stability and thermal stability, mild reaction conditions, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
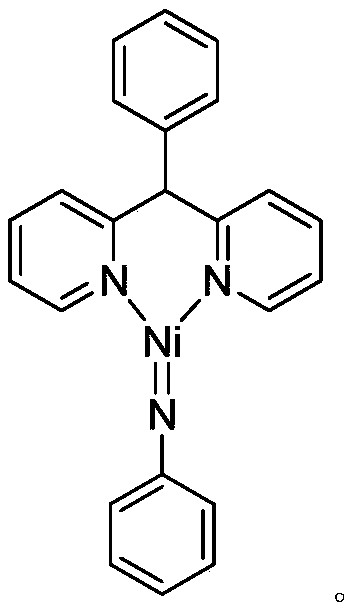
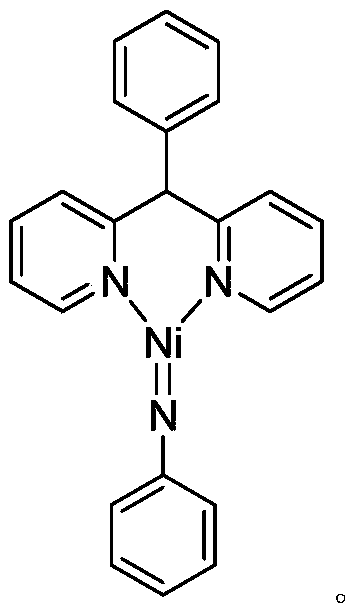
[0026] Synthesis of divalent nickel-imine complexes containing phenyldipyridine nickel-nitrogen double bond structure:
[0027]
[0028] At room temperature, the phenylbipyridine ligand C 17 h 14 N 2 (246.3mg, 1.0mmol) of toluene solution was added dropwise to Ni(COD) 2 (220.0mg, 0.80mmol) in tetrahydrofuran solution, stirred at this temperature for 3 hours, then benzene azide PhN 3 (114.2 mg, 0.96 mmol) was added to the reaction system for an additional 3 hours. After the reaction is over, let it stand and filter, and dry the solvent under reduced pressure, and the obtained crude product is subjected to column chromatography separation (petroleum ether / ethyl acetate (v / v)=5:1) to obtain the light yellow target product divalent nickel imine complex C 23 h 19 N 3 Ni (243.9 mg, 77% yield). Elemental analysis theoretical value: C 69.74, H 4.83, N 10.61; experimental value: C 69.68, H 4.93, N 10.55.
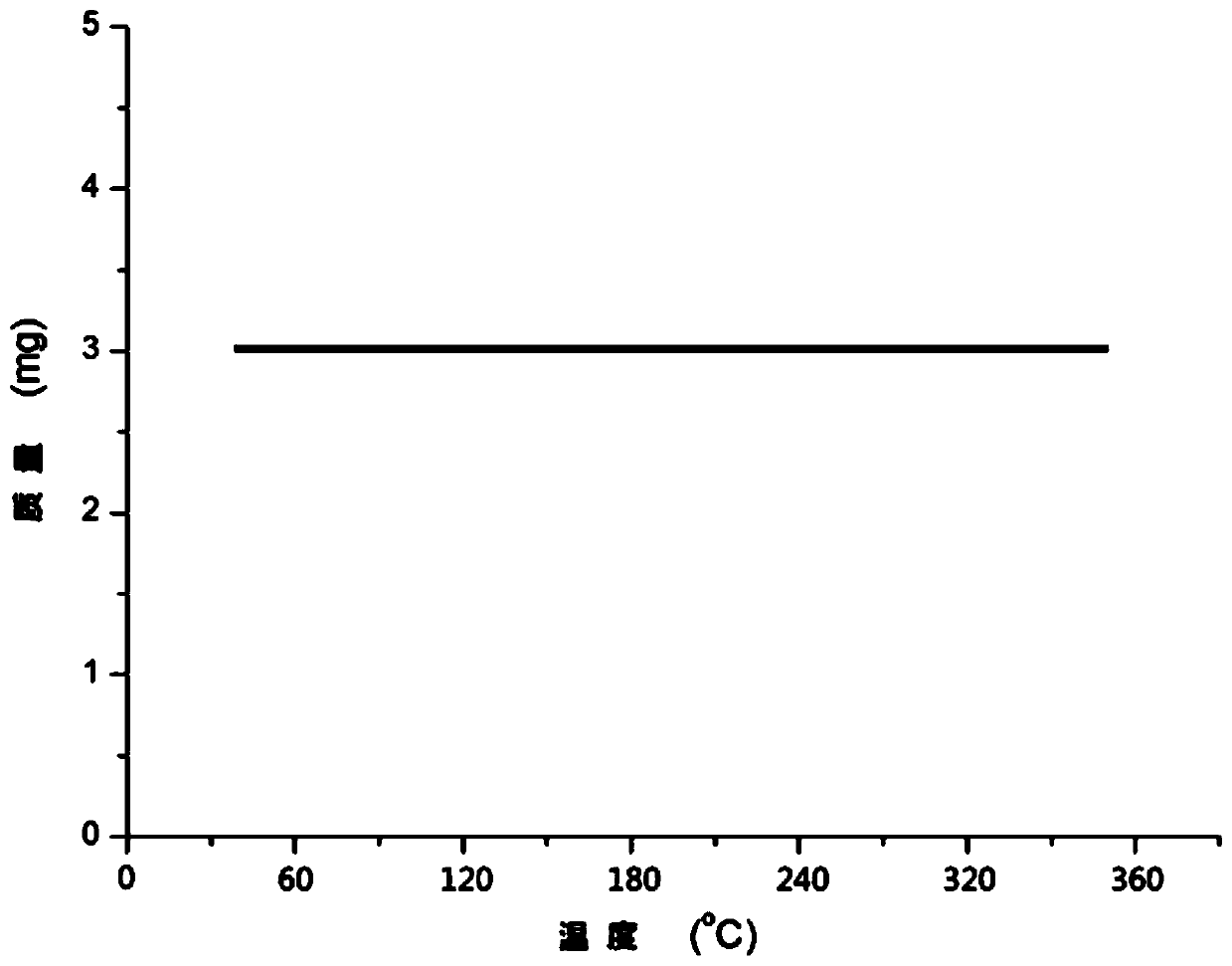
[0029] Take 3.0mg compound and carry out thermogravimetric experimen...
Embodiment 2
[0031] Divalent nickel-imine complexes catalyze the reverse Malanobilic amination of styrene:
[0032]
[0033] The nickel imine complex prepared in Example 1 is used as a catalyst to catalyze the reverse Malanobiline amination reaction of olefins: add toluene containing nickel imine complex (0.001 mmol) to styrene (1 mmol) and aniline (1 mmol) solution, the reaction temperature is 25°C, and the reaction time is 60 minutes. After the completion, the concentrated reaction solution is directly separated by silica gel column chromatography, dried until the mass remains constant, and the corresponding amine compound C is obtained. 14 h 15 N, yield 88%, elemental analysis: C 85.24, H 7.66, N 7.10 (theoretical); C85.31, H 7.69, N 7.02 (actual).
Embodiment 3
[0035] Divalent nickel-imine complexes catalyze the reverse Malanobilic amination of styrene:
[0036]
[0037] The nickel imine complex prepared in Example 1 is used as a catalyst to catalyze the anti-Martensio amination reaction of olefins: add nickel imine complex (0.002mmol) to styrene (1mmol) and methylaniline (1mmol) toluene solution, the reaction temperature is 25°C, and the reaction time is 100 minutes. After the end, the concentrated reaction solution is directly separated by silica gel column chromatography, dried until the quality remains unchanged, and the corresponding amine compound C is obtained. 15 h 17 N, yield 97%, elemental analysis: C 85.26, H 8.11, N 6.63 (theoretical); C 85.35, H 8.03, N 6.60 (actual).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com