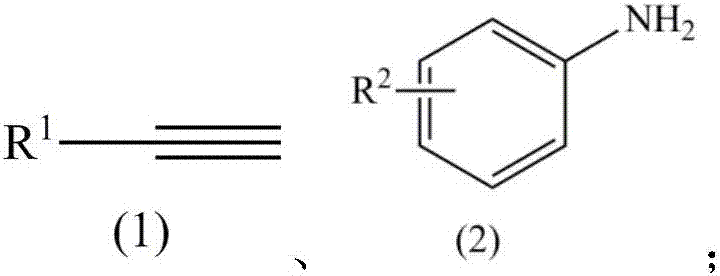
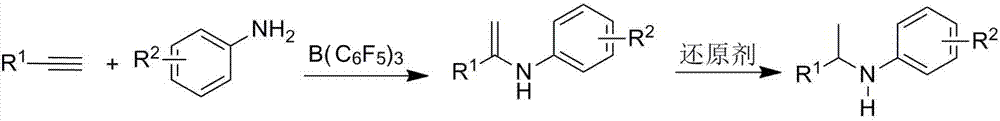
Method for catalyzing intermolecular hydroamination reaction of alkyne and amine
An intermolecular, hydroamination technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino compounds by condensation/addition reactions, etc. The effect of mild reaction conditions, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
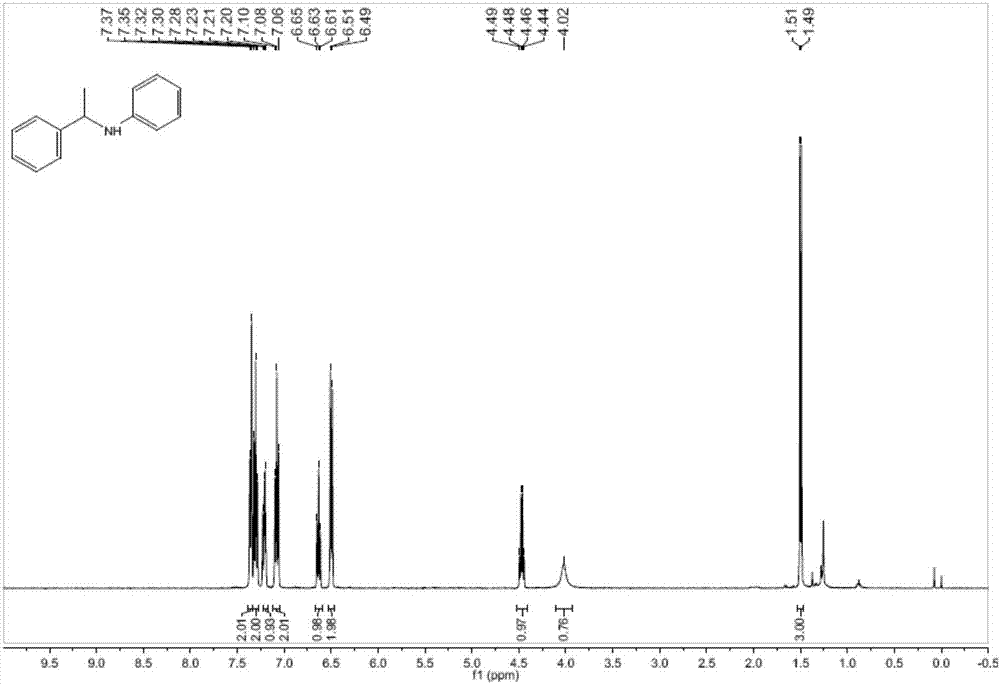
[0035] The present embodiment provides a kind of utilization 15mol% B (C 6 f 5 ) 3 The method for catalyzing the intermolecular hydroamination reaction of phenylacetylene and aniline under the condition of 100 DEG C, the specific steps are as follows:
[0036] 39mg (0.075mmol) of B (C 6 f 5 ) 3 Dissolve in 2mL of chlorobenzene, add 0.22mL (2.0mmol) phenylacetylene and 0.046mL (0.5mmol) aniline to the solution after the reaction is complete, seal the tube, under the conditions of anhydrous, anaerobic and argon protection, 100 Under the condition of ℃, react for 24h. Open the bottle after the reaction temperature is down to room temperature, add 60mg (1.5mmol) LiAlH in the reaction bottle 4 , reacted at 70°C for 2h, quenched the reaction with 2M NaOH, suction filtered, separated, took the organic phase, concentrated it and adsorbed it with basic alumina, and column chromatography (ethyl acetate / petroleum ether=1 / 100 ), to obtain the target product 97mg, yield 99%. The st...
Embodiment 2
[0039] The present embodiment provides a kind of utilization 20mol%B (C 6 f 5 ) 3 The method for catalyzing the intermolecular hydroamination reaction of phenylacetylene and aniline under the condition of 100 DEG C, the specific steps are as follows:
[0040] 51mg (0.1mmol) of B (C 6 f 5 ) 3 Dissolve in 2mL of chlorobenzene, add 0.22mL (2.0mmol) phenylacetylene and 0.046mL (0.5mmol) aniline to the solution after the reaction is complete, seal the tube, under the conditions of anhydrous, anaerobic and argon protection, 100 Under the condition of ℃, react for 12h. Open the bottle after the reaction temperature is down to room temperature, add 60mg (1.5mmol) LiAlH in the reaction bottle 4 , reacted at 70°C for 2h, quenched the reaction with 2M NaOH, suction filtered, separated, took the organic phase, concentrated it and adsorbed it with basic alumina, and column chromatography (ethyl acetate / petroleum ether=1 / 100 ), to obtain the target product 95mg, yield 97%. The struc...
Embodiment 3
[0048] The present embodiment provides a kind of utilization 15mol% B (C 6 f 5 ) 3 The method for catalyzing the intermolecular hydroamination reaction of p-methylphenylacetylene and aniline under the condition of 100 DEG C, the specific steps are as follows:
[0049] 39mg (0.075mmol) of B (C 6 f 5 ) 3 Dissolve in 2mL of chlorobenzene, add 0.25mL (2.0mmol) p-tolueneacetylene and 0.046mL (0.5mmol) aniline to the solution after the reaction is complete, seal the tube, and use the conditions of anhydrous, anaerobic and argon protection Under the condition of 100°C, react for 12h. Open the bottle after the reaction temperature is down to room temperature, add 60mg (1.5mmol) LiAlH in the reaction bottle 4 , reacted at 70°C for 2h, quenched the reaction with 2M NaOH, suction filtered, separated, took the organic phase, concentrated it and adsorbed it with basic alumina, and column chromatography (ethyl acetate / petroleum ether=1 / 100 ), to obtain the target product 100mg, yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com