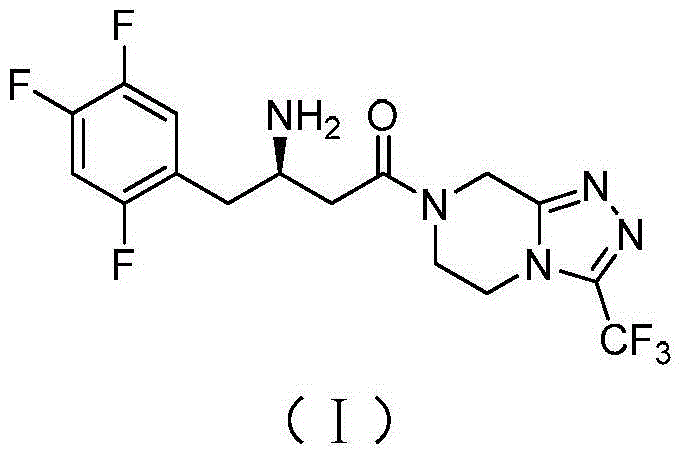
Synthetic method of sitagliptin and salt thereof
A synthesis method and technology of sitagliptin are applied in the field of synthesis of sitagliptin and its salts, which can solve problems such as platinum-carbon flammability yield, avoid the use of strong bases or Grignard reagents, have high purity, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
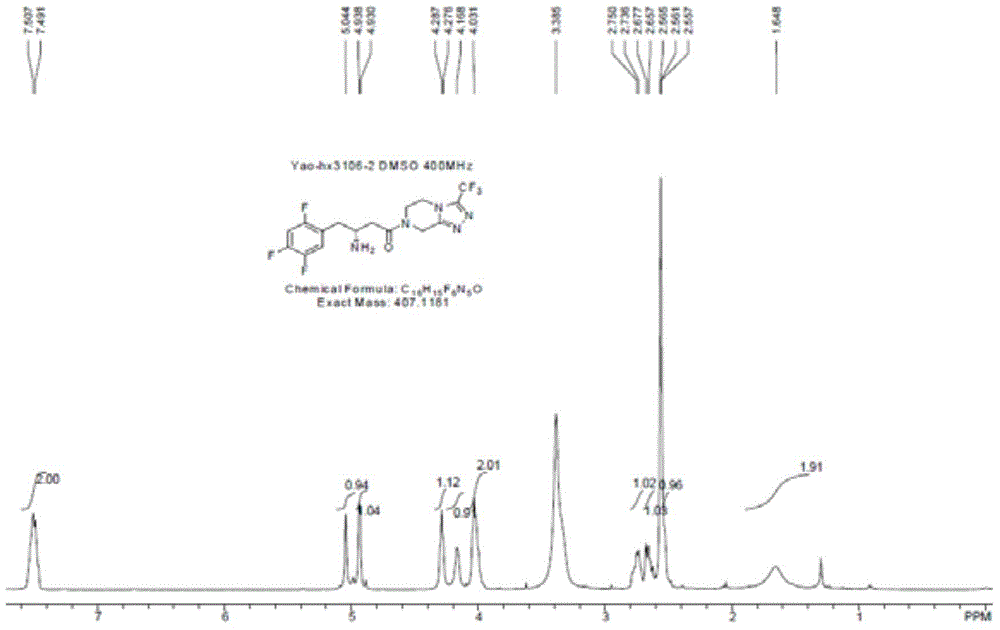
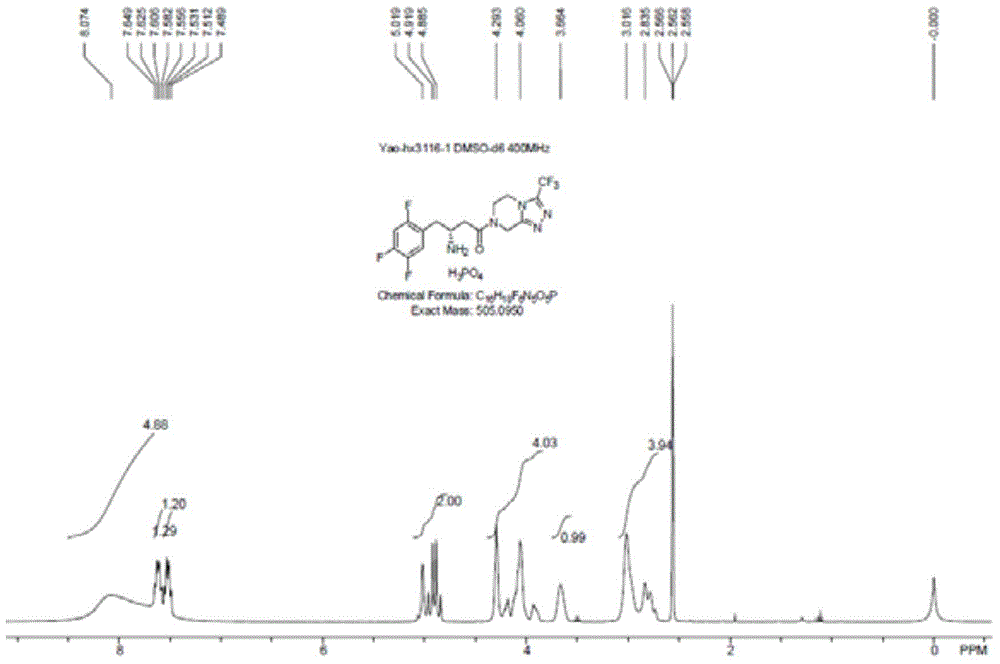
[0062] Dissolve 2,4,5-trifluorophenylacetic acid (CAS: 209995-38-0, 1.9 g, 10 mmol) in methanol (20 mL), and add a few drops of concentrated sulfuric acid. After heating to reflux for three hours, it was cooled to room temperature, and the solvent was evaporated by a rotary evaporator. The residue was dissolved in dichloromethane (20 mL), washed with sodium bicarbonate (1M) solution, and washed with water. The organic phase was dried and evaporated to dryness with a rotary evaporator to obtain an oily substance.
[0063] The oil obtained above was dissolved in tetrahydrofuran (40 mL), and DIBAL-H (1M dissolved in toluene, 20 mL, 20 mmol) was added dropwise in an ice-water bath, and reacted overnight at room temperature. The next day, add water (20 mL) to quench the reaction under an ice-water bath, and adjust the pH value to 2-3 with concentrated hydrochloric acid. Extracted three times with dichloromethane (10 mL), combined the organic phases, dried and evaporated to drynes...
Embodiment 2
[0067] 2,4,5-Trifluorophenylacetic acid (CAS: 209995-38-0, 1.9 g, 10 mmol) was dissolved in a solution of tetrahydrofuran (40 mL), and then added dropwise to 1M borane tetrahydrofuran under ice-water bath at 0-5°C solution (12mL, 1.2mmol), the dropwise addition was completed, and the reaction was carried out overnight at room temperature. The next day, the reaction was quenched by adding water (20 mL) under an ice-water bath. Extracted three times with dichloromethane (10 mL), combined the organic phases, dried and evaporated to dryness with a rotary evaporator to obtain a colorless liquid (yield: 89%, purity: 98%) and directly used in the next step.
Embodiment 3
[0069] 2,4,5-Trifluorophenylethanol (1.76g, 10mmol) prepared in Example 1 was dissolved in acetonitrile (40mL), and IBX (Chinese name: o-iodobenzoic acid, CAS: 64297-64-9) was added ( 2.8 g, 10 mmol). After reflux for two hours, the reaction solution was cooled to room temperature, and the solid was removed by filtration. The filtrate was evaporated to dryness with a rotary evaporator, dissolved in diethyl ether, and washed with water. Separate the liquid, dry the organic phase, and evaporate to dryness with a rotary evaporator to obtain a yellow oil (yield: 76%, purity: 92%), which is directly used for the next step. product by 1 Confirmed by H-NMR and TLC. The reaction formula is as follows:
[0070]
[0071] 1 The H-NMR data are as follows: 1 HNMR (CDCl 3 ,400MH Z ): 9.77 (1H, m), 7.69~7.45 (1H, m), 7.38~7.31 (1H, m), 3.74 (2H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com