Sequence-controllable linear/hyperbranched polymer prepared through metal-free catalyzed multi-component polymerization, and preparation method and application thereof
A technology of hyperbranched polymers and metal-free catalysis, which is applied in the fields of fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of high cost and demanding requirements, and achieve the effects of good film formation, mild conditions and high polymer yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
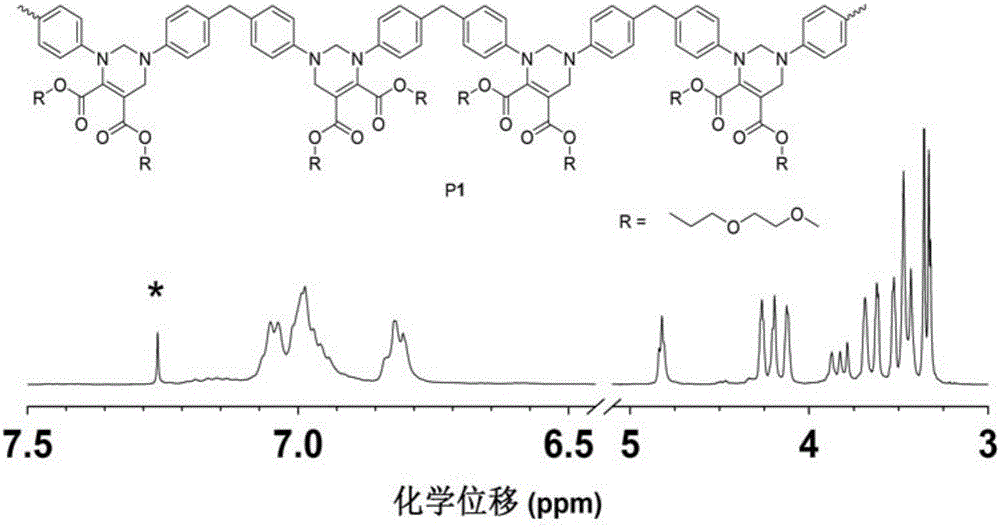
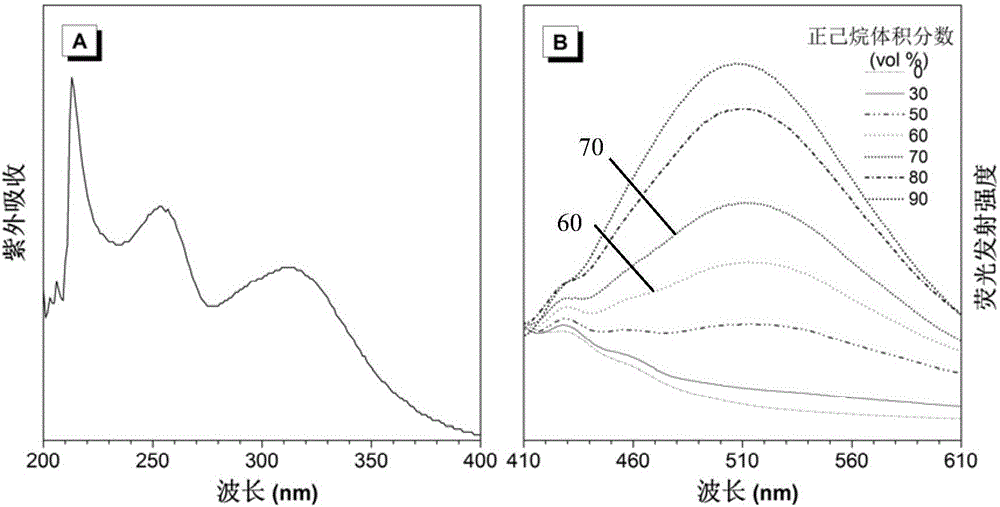
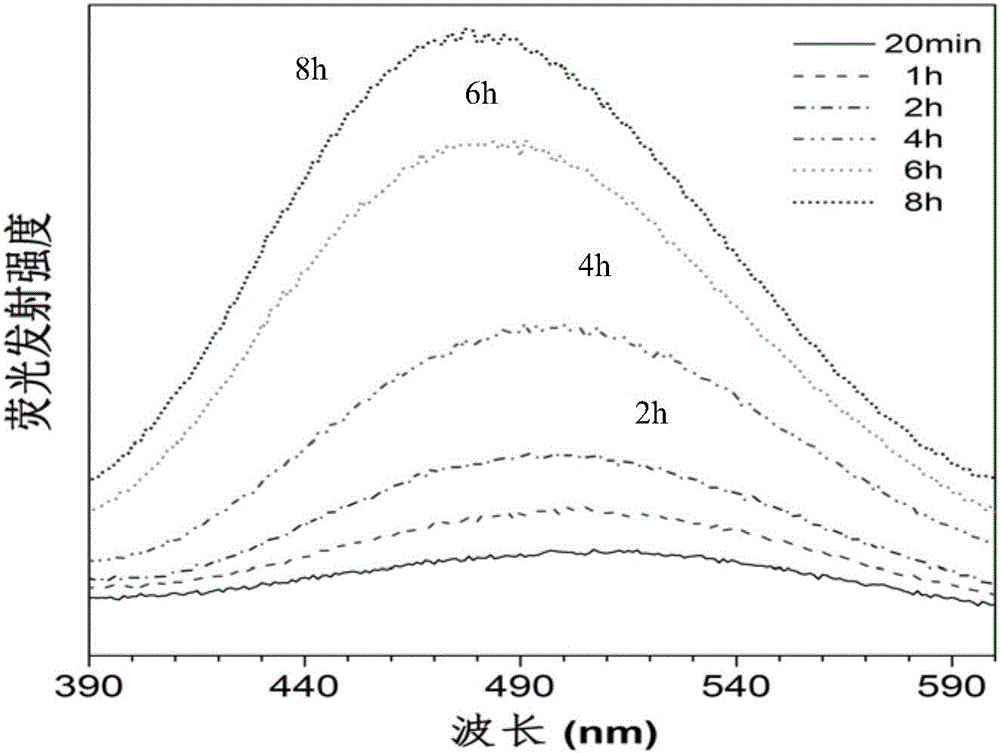
[0056] This example is a method for preparing polytetrahydropyrimidine by metal-free multi-component polymerization of alkynes, aldehydes and amines. The specific preparation steps are as follows:
[0057] The monomer raw material 4,4'-diaminodiphenylmethane (M1) is directly ordered from Alfa Aesar, and the monomer butynedioic acid dimethyl carbitate (M2) is obtained according to the literature [Macromolecules 2012,45,3687- 3694] prepared by the disclosed method; monomer raw material mass percent is 38% formaldehyde solution (M3) directly ordered from TCI company.
[0058] Add dimethyl carbityl butyndioate (0.1910g, 0.6mmol), 4,4'-diaminodiphenylmethane (0.1190g, 0.6mmol), and 2ml methanol into the polymerization tube, at room temperature (25°C) React for 30min; then add formaldehyde solution (136ul, 1.8mmol) and acetic acid (206ul, 1.2mmol) with a mass percentage of 38%, respectively, and continue to react at room temperature (25°C) for 16h; under stirring conditions, the rea...
Embodiment 2
[0067] This example is a method for preparing polydihydropyrrole by metal-free catalyzed multi-component polymerization of alkynes, aldehydes and amines. The specific preparation steps are as follows:
[0068] The monomer raw material 4,4'-diaminodiphenylmethane (M1) is directly ordered from Alfa Aesar, and the monomer butynedioic acid dimethyl carbitate (M2) is obtained according to the literature [Macromolecules 2012,45,3687- 3694] prepared by the disclosed method; monomer raw material mass percent is 38% formaldehyde solution (M3) directly ordered from TCI company.
[0069] Add dimethyl carbityl butyndioate (0.1910g, 0.6mmol), 4,4'-diaminodiphenylmethane (0.0595g, 0.3mmol), and 2ml of ethanol into the polymerization tube, at room temperature (25°C) React for 30min; then add 4,4'-diaminodiphenylmethane (0.0595g, 0.3mmol), 38% by mass aqueous formaldehyde solution (45ul, 0.6mmol), acetic acid (206ul, 1.2mmol), at 70 The reaction was continued at ℃ for 16 h; under the conditi...
Embodiment 3
[0073] This example is a method for preparing polydihydropyrrole by metal-free catalyzed multi-component polymerization of alkynes, aldehydes and amines. The specific preparation steps are as follows:
[0074] Monomer raw material p-phenylenediamine (M4), phenylamine (M5), terephthalaldehyde (M6) are directly ordered from Alfa Aesar company, and monomer butynedioic acid dimethyl carbitate (M2) is according to document [ Prepared by the disclosed method of Macromolecules 2012,45,3687-3694].
[0075] Add dimethyl carbityl butynedioate (0.1910g, 0.6mmol), p-phenylenediamine (0.0324g, 0.3mmol), and 2ml of ethanol into the polymerization tube, and react at room temperature for 30min; then add phenylamine (0.0559g , 0.6mmol), terephthalaldehyde (0.0402g, 0.3mmol), acetic acid (206ul, 1.2mmol), continue to react at room temperature for 16h; under the condition of stirring, the solution after the reaction is added dropwise in n-hexane, After standing still for 12 h, the precipitate w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com