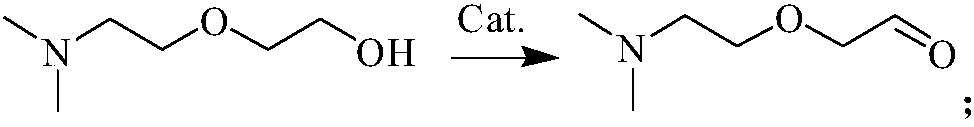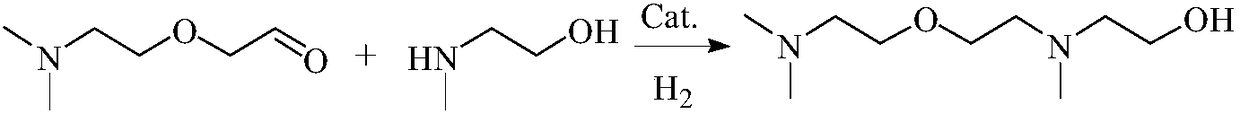Synthesis method of N, N, N'-trimethyl-N'-hydroxyethyl diaminoethyl ether
A technology of bisaminoethyl ether and dimethylaminoethoxyethanol is applied in the synthesis field of polyurethane catalyst, can solve the problems of unfavorable industrial production, low atom economy, low application value, etc. Product yield, high atom economy, and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, a kind of synthetic method of 2-[2-(dimethylamino)ethoxyl]acetaldehyde, take dimethylaminoethoxyethanol as raw material, 2,2,6,6-tetramethylpiperene Pyroxyl-FeCl 3 - tert-butyl nitrite catalysis:
[0045] 13.3g of dimethylaminoethoxyethanol, 1.248g of 2,2,6,6-tetramethylpiperidine oxide, 0.824g of tert-butyl nitrite, 1.248g of FeCl were put into a 100ml autoclave 3 and 15ml of dichloromethane.
[0046] Fill the kettle with oxygen to 0.6MPa at room temperature, heat to 80°C and stir for 8h. After the reaction was finished, it was lowered to room temperature, and the pressure inside the kettle was 0.2 MPa (20° C.). Then the still liquid is subjected to the following post-treatment: filtration, and the resulting filtrate is distilled under reduced pressure (25 Torr pressure, collecting fractions at 75-80° C.) to obtain 8.02 g of colorless and transparent 2-[2-(dimethylamino)ethoxy base] acetaldehyde. The conversion rate of the raw material obtained after...
Embodiment 1-1~ Embodiment 1-9
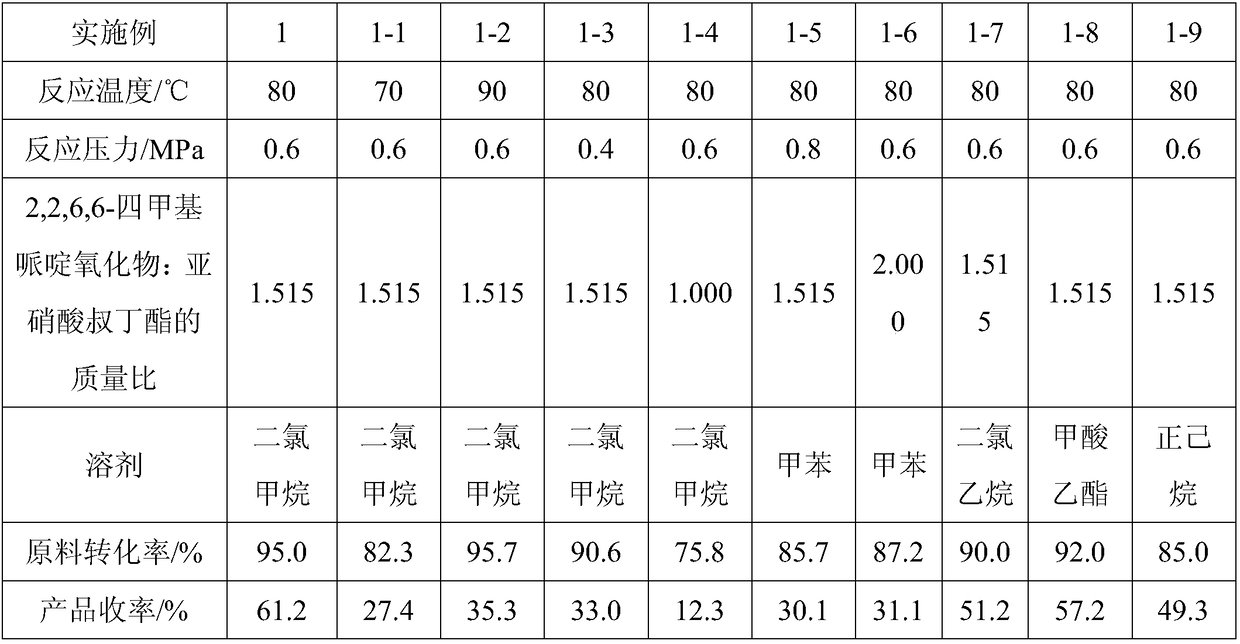
[0048] Change the following reaction conditions in embodiment 1: change temperature of reaction in embodiment 1, reaction pressure, catalyst ratio (change the mass ratio of 2,2,6,6-tetramethyl piperidine oxide and tert-butyl nitrite , the amount of 2,2,6,6-tetramethylpiperidinium oxide remains unchanged), the kind of solvent; all the other are equal to embodiment 1 (FeCl 3 The dosage ratio remains unchanged); thus, Examples 1-1 to 1-9 are obtained, and the total yield is shown in Table 1.
[0049] Table 1
[0050]
[0051]Embodiment 1-10~1-17, change the following reaction conditions in embodiment 1: change the reaction time in embodiment 1, catalyst 2,2,6,6-tetramethylpiperidinium oxide accounts for two Methylaminoethoxyethanol mass ratio, all the other are equal to embodiment 1 (that is, the mass ratio of 2,2,6,6-tetramethylpiperidine oxide and tert-butyl nitrite remains constant, FeCl 3 The mass ratio to 2,2,6,6-tetramethylpiperidinium oxide remains unchanged); thus, E...
Embodiment 2
[0054] Example 2, 2-[2-(Dimethylamino)ethoxy]acetaldehyde and methylethanolamine hydrogenation amination to prepare N,N,N'-trimethyl-N'-hydroxyethylbisaminoethyl Base ether:
[0055] 13.1 g (0.1 mol) of 2-[2-(dimethylamino)ethoxy]acetaldehyde, 8.3 g (0.1 mol) of N-methylethanolamine, 1.3 g of Raney Ni and 15 ml of methanol were put into a 100 ml autoclave. At room temperature (about 20°C), fill the kettle with hydrogen to 2.0MPa, heat to 120°C and stir for 8h, keeping the pressure inside the kettle (adjusted by hydrogen) at 2.0-2.2MPa.
[0056] After the reaction was completed, it was lowered to room temperature and filtered. Gained filter cake is dropped into concentrated sodium hydroxide solution (mass concentration is 40% sodium hydroxide aqueous solution) immediately and soaks at least 30 minutes, then washes repeatedly with clear water (to washing liquid pH is about 8), the Raney Ni of gained can be recycle. The filtrate was analyzed by gas chromatography, and the conv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com