Synthesis method of trimetazidine hydrochloride
A technology of trimetazidine hydrochloride and its synthesis method, which is applied in the field of synthesis of antiangina pectoris drugs, can solve problems such as unfavorable production, cumbersome operation steps, and unsuitability for industrial production, so as to improve yield and purity, reduce production cost, and easily The effect of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
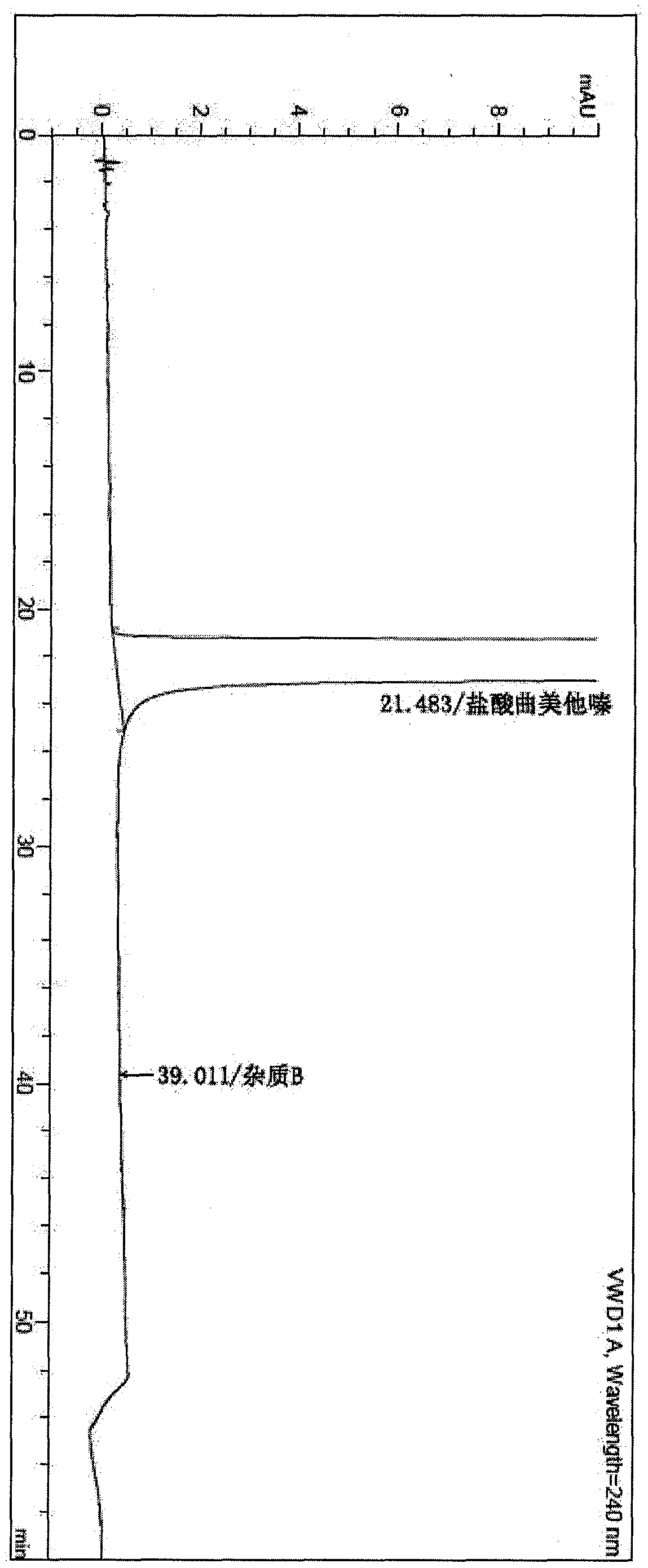
[0039] Add 500kg of ethanol, 120kg of 2,3,4-trimethoxybenzaldehyde, 150kg of anhydrous piperazine, and 2kg of Lindella catalyst (Pd-CaCO3-PbO containing 10% palladium) to the 2000L hydrogenation reactor, and the addition is completed. The hydrogen pressure was 2.0MPa, and the temperature was raised to 60°C and kept stirring for 16 hours, during which the hydrogen pressure was controlled at 2.0Mpa until the reaction was complete. The reaction solution was lowered to room temperature, filtered to remove the Lindella catalyst, concentrated under reduced pressure at 30-40°C until there was no distillate, added 1000kg of acetone, stirred for 10 minutes, added concentrated hydrochloric acid to 125kg, cooled to 10°C for crystallization and stirred for 5 hours, and centrifuged to obtain Trimetazidine hydrochloride was 191.3kg, the yield was 92.5%, and the purity was 100%, and the trimetazidine hydrochloride impurity B was not detected.
Embodiment 2
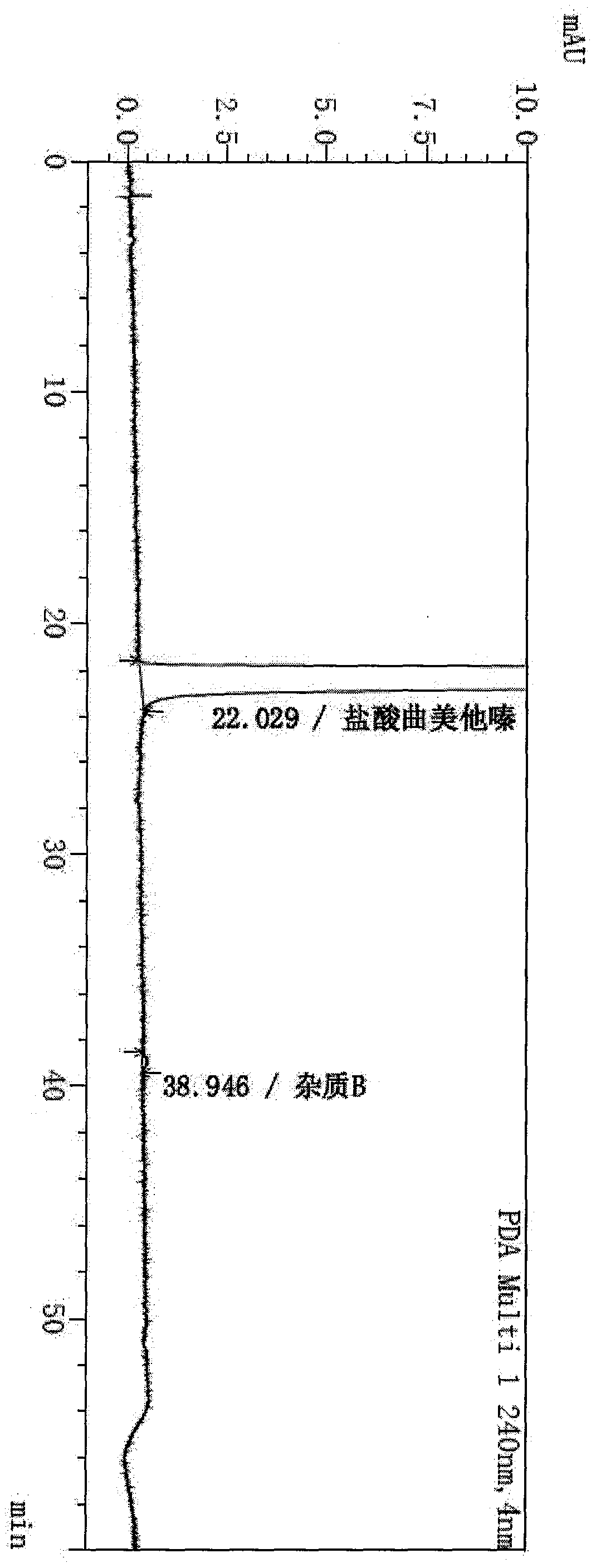
[0041] In the 2000L hydrogenation reactor, add 450kg methanol, 120kg 2,3,4-trimethoxybenzaldehyde, 210kg anhydrous piperazine, 2.4kg Lindela catalyst (Pd-CaCO3-PbAc2 containing palladium 5%), finish . The hydrogen pressure was 2.0MPa, and the temperature was raised to 65°C and kept stirring for 16 hours, during which the hydrogen pressure was controlled at 1.0Mpa until the reaction was complete. The reaction solution was lowered to room temperature, filtered to remove the Lindella catalyst, concentrated under reduced pressure at 30-40°C until there was no distillate, added 1000kg of acetone, stirred for 10 minutes, added 125kg of concentrated hydrochloric acid, cooled to 20°C for crystallization and stirred for 5 hours, and centrifuged to obtain Trimetazidine hydrochloride was 189.6 kg, the yield was 91.7%, and the purity was 99.9%, and trimetazidine hydrochloride impurity B was not detected.
Embodiment 3
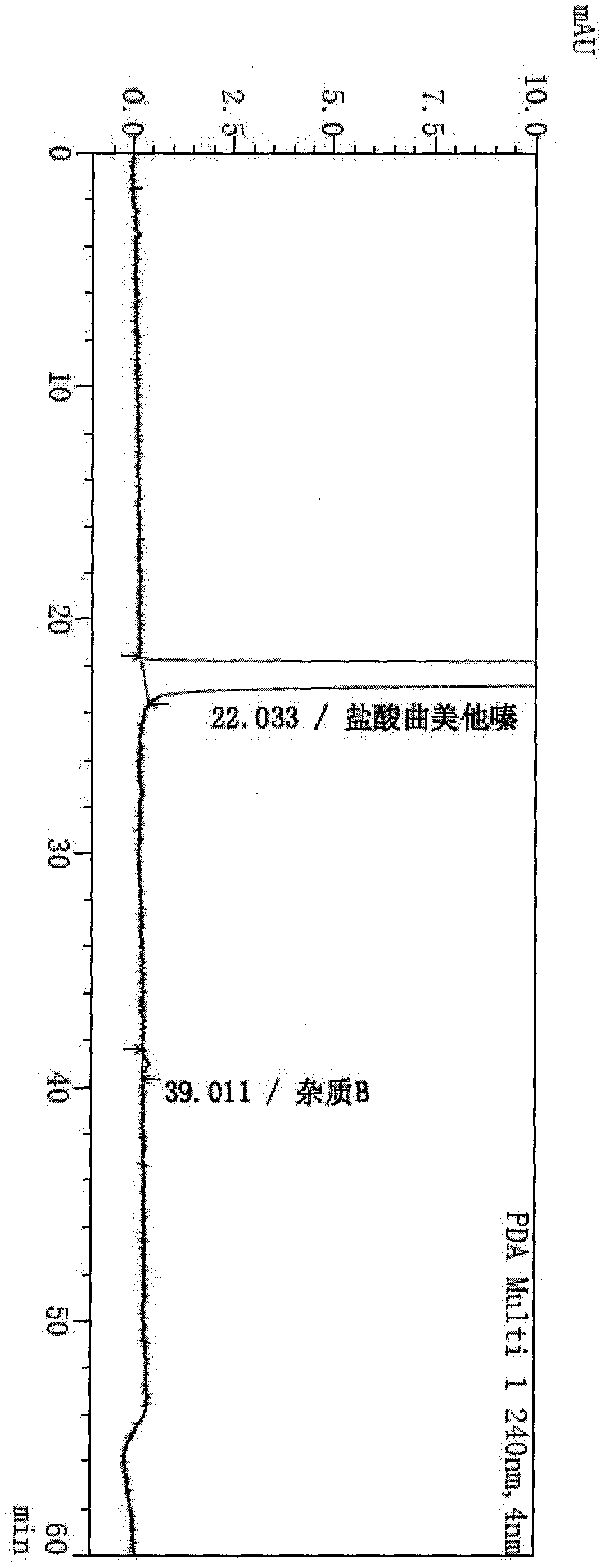
[0043] In the 2000L hydrogenation reactor, add 550kg Virahol, 120kg 2,3,4-trimethoxybenzaldehyde successively, 184kg anhydrous piperazine, 3.6kg Lindela catalyst (Pd-CaCO3-PbO contains palladium 7%), Gabi. The hydrogen pressure was 2.0MPa, and the temperature was raised to 50°C and kept stirring for 17 hours, during which the hydrogen pressure was controlled at 2.5Mpa until the reaction was complete. The reaction solution was lowered to room temperature, filtered to remove the Lindella catalyst, concentrated under reduced pressure at 30-40°C until there was no distillate, added 1000kg of ethyl acetate, stirred for 10 minutes, added 125kg of concentrated hydrochloric acid, cooled to 15°C for crystallization and stirred for 5 hours. 190.5 kg of trimetazidine hydrochloride was obtained by centrifugation, with a yield of 92.1% and a purity of 99.8%, and trimetazidine hydrochloride impurity B was not detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com