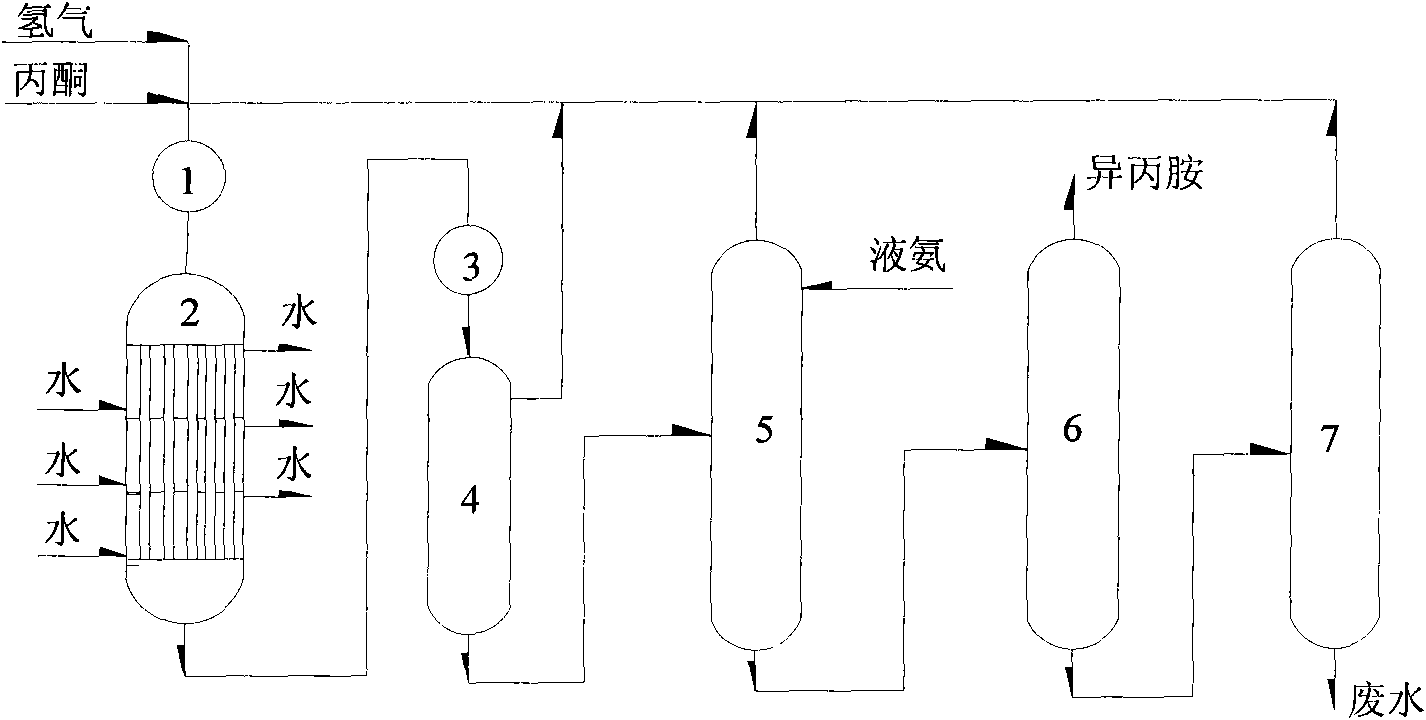
Method for producing isopropylamine
A technology of isopropylamine and diisopropylamine, which is applied in the field of isopropylamine production, can solve the problems that diisopropylamine cannot be effectively converted into isopropylamine, the operation difficulty of the rectification system, and the increase of production cost, etc., and the method is simple, feasible, and considerable economic benefits , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This example is used to illustrate that acetone, diisopropylamine, and isopropanol are converted into isopropylamine at different reaction temperatures.
[0032] In the laboratory, a fixed-bed reactor was used, and the isopropylamine catalyst produced by Beijing Research Institute of Chemical Industry was used, and the filling volume was 50 ml to carry out the gas phase reaction.
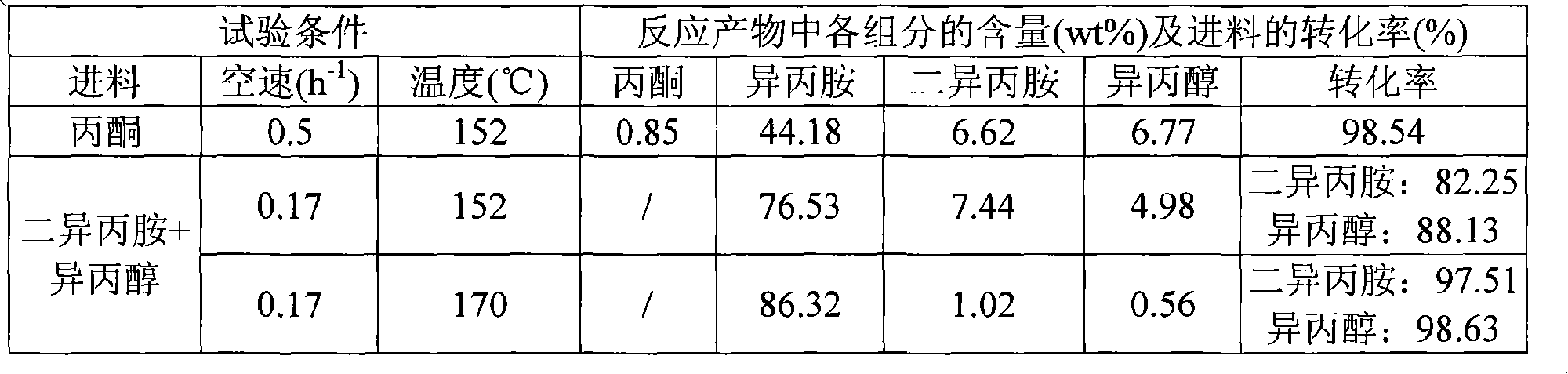
[0033] Experiment 1: Use acetone as raw material to carry out hydrogenation reaction to synthesize isopropylamine. The reaction conditions are: the volume space velocity of liquid acetone is 0.5h -1 , the pressure is normal pressure, the reaction temperature is 152°C, and the molar ratio of acetone:hydrogen:ammonia is 1:3:3. The test results are listed in Table 1.
[0034] Test 2: In order to simulate the material in the industrial plant, a mixture of 50 wt% of diisopropylamine and isopropanol is used as a raw material. In the presence of hydrogen, they react with ammonia and convert to isop...
Embodiment 2
[0039] This embodiment is used to explain the hydroamination reaction of the present invention with staged temperature control.
[0040] In order to simulate the segmental temperature control of the reactor in the industrial device, the reactor is divided into two sections in the laboratory, that is, two sections of beds, each section is filled with 50 ml of isopropylamine catalyst produced by Beijing Research Institute of Chemical Industry, and the temperature of the two sections is controlled respectively. Perform a gas phase reaction.
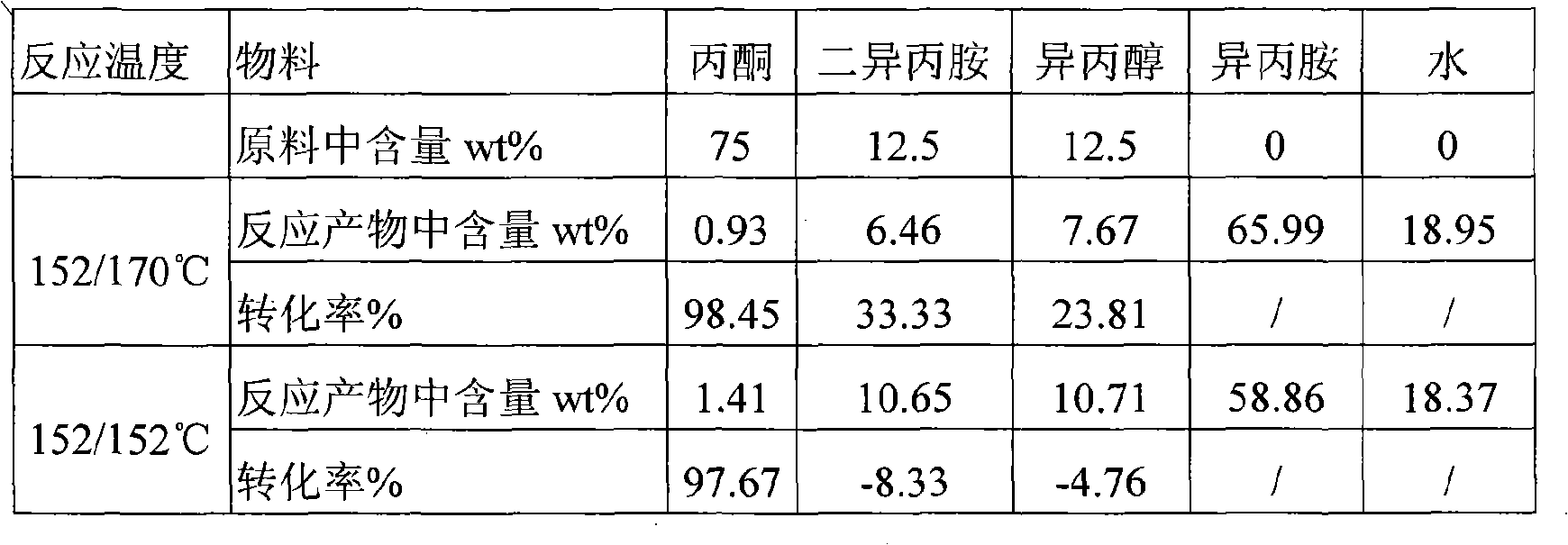
[0041] The feed is a mixture containing 75wt% acetone, 12.5wt% diisopropylamine and 12.5wt% isopropanol, and the liquid volume space velocity of acetone is 0.5h -1 . The reaction temperature of the first section bed is controlled at 152°C, the reaction temperature of the second section bed is controlled at 170°C, and the pressure is normal pressure, (acetone+diisopropylamine+isopropanol): hydrogen: the mol ratio of ammonia is 1:3:3. The c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com