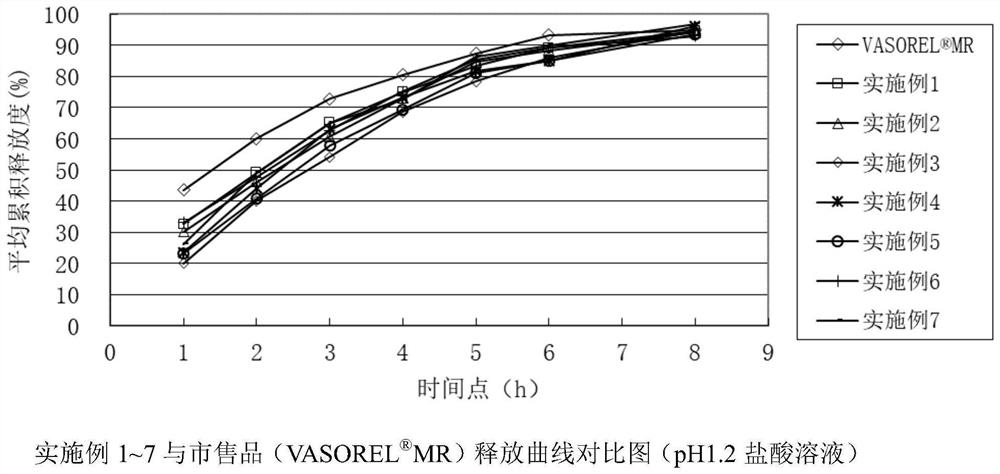
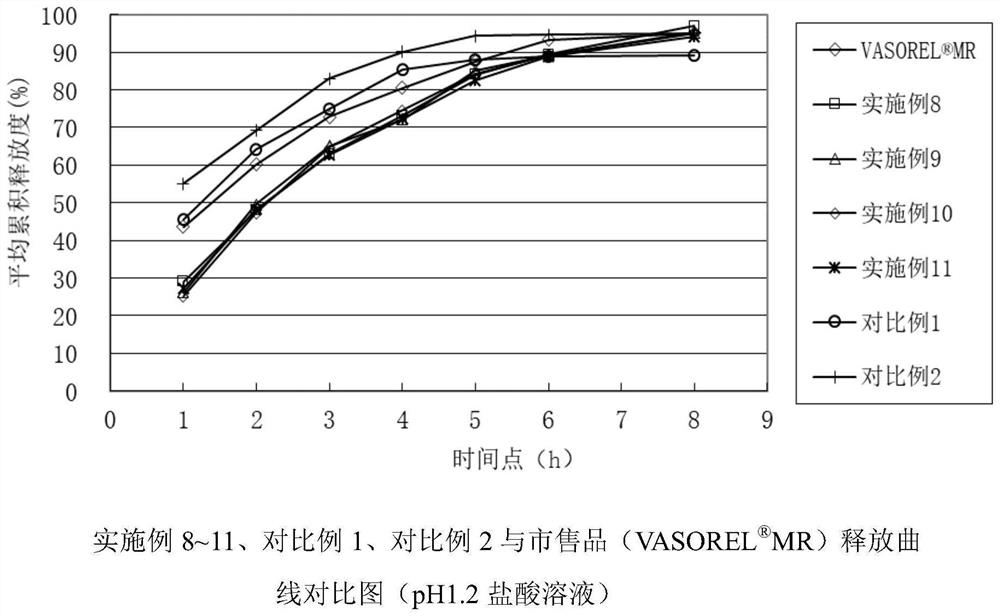
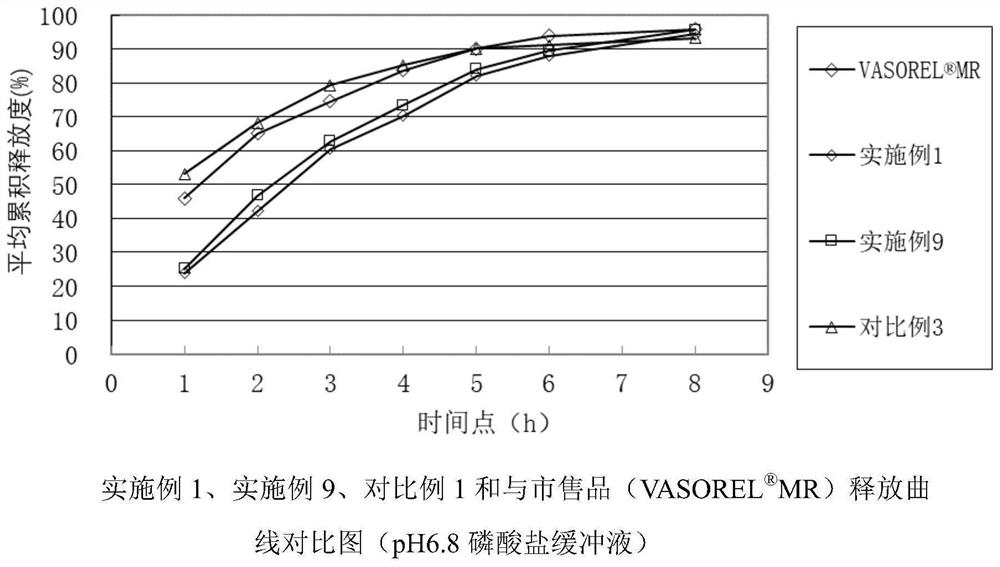
A kind of trimetazidine hydrochloride sustained-release tablet and preparation method thereof
A technology of trimetazidine hydrochloride and sustained-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc. Release effects, unstable quality of sustained-release tablets, etc., to achieve the effects of reducing side effects, improving drug safety, and reducing the number of medications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. The composition of the prescription of trimetazidine hydrochloride sustained-release tablets: the specification is 35 mg, and the prescription quantity is 1000 tablets.
[0040]
[0041] Preparation:
[0042] Pretreatment: pass the raw and auxiliary materials through a 40-mesh sieve for pretreatment; mixing: weigh the prescription amount of trimetazidine hydrochloride and calcium hydrogen phosphate dihydrate and mix them evenly in a hopper mixer, then mix hypromellose, carbopol Add 974P and Eudragit L100 to the above materials and mix evenly. The above materials and colloidal silicon dioxide are mixed twice through a 30-mesh sieve and placed in a hopper mixer. Magnesium stearate is added and mixed evenly to obtain a total blended powder; tableting : Put the mixed powder in a rotary tablet press for tableting to obtain plain tablets; Coating: Put the plain tablets in a high-efficiency coating machine for film coating.
Embodiment 2
[0043] Example 2. The composition of the prescription of trimetazidine hydrochloride sustained-release tablets: the specification is 35 mg, and the prescription quantity is 1000 tablets.
[0044]
[0045] The preparation method is as follows:
[0046]Pretreatment: pass the raw and auxiliary materials through a 40-mesh sieve for pretreatment; mixing: weigh the prescription amount of trimetazidine hydrochloride and calcium hydrogen phosphate dihydrate and mix them evenly in a hopper mixer, then mix hypromellose, carbopol Mu 934P and Eudragit S100 were added to the above materials and mixed evenly. The above materials were mixed with colloidal silicon dioxide through a 30-mesh sieve twice, then placed in a hopper mixer, and magnesium stearate was added to mix evenly to obtain a total blended powder; tableting : Put the mixed powder in a rotary tablet press for tableting to obtain plain tablets; Coating: Put the plain tablets in a high-efficiency coating machine for film coatin...
Embodiment 3
[0047] Example 3. The composition of the prescription of trimetazidine hydrochloride sustained-release tablets: the specification is 35 mg, and the prescription quantity is 1000 tablets.
[0048]
[0049]
[0050] The preparation method is as follows:
[0051] Pretreatment: pass the raw and auxiliary materials through a 40-mesh sieve for pretreatment; mixing: weigh the prescription amount of trimetazidine hydrochloride and calcium hydrogen phosphate dihydrate and mix them evenly in a hopper mixer, then mix hypromellose, carbopol Add 974P and Eudragit S100 to the above materials and mix evenly. The above materials and colloidal silicon dioxide are mixed twice through a 30-mesh sieve and placed in a hopper mixer. Magnesium stearate is added and mixed evenly to obtain a total blended powder; tableting : Put the mixed powder in a rotary tablet press for tableting to obtain plain tablets; Coating: Put the plain tablets in a high-efficiency coating machine for film coating.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com