Trimetazidine hydrochloride single-layer osmotic pump controlled release tablet and preparation method thereof
A technology of trimetazidine hydrochloride and single-layer osmotic pump is applied in the field of trimetazidine hydrochloride single-layer osmotic pump controlled-release tablet and its preparation field, which can solve the problems of high equipment and process requirements, complex composition of auxiliary materials, and punching equipment. It is impossible to distinguish between the drug-containing layer and the booster layer, so as to achieve the effect of rapid onset and reduction of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
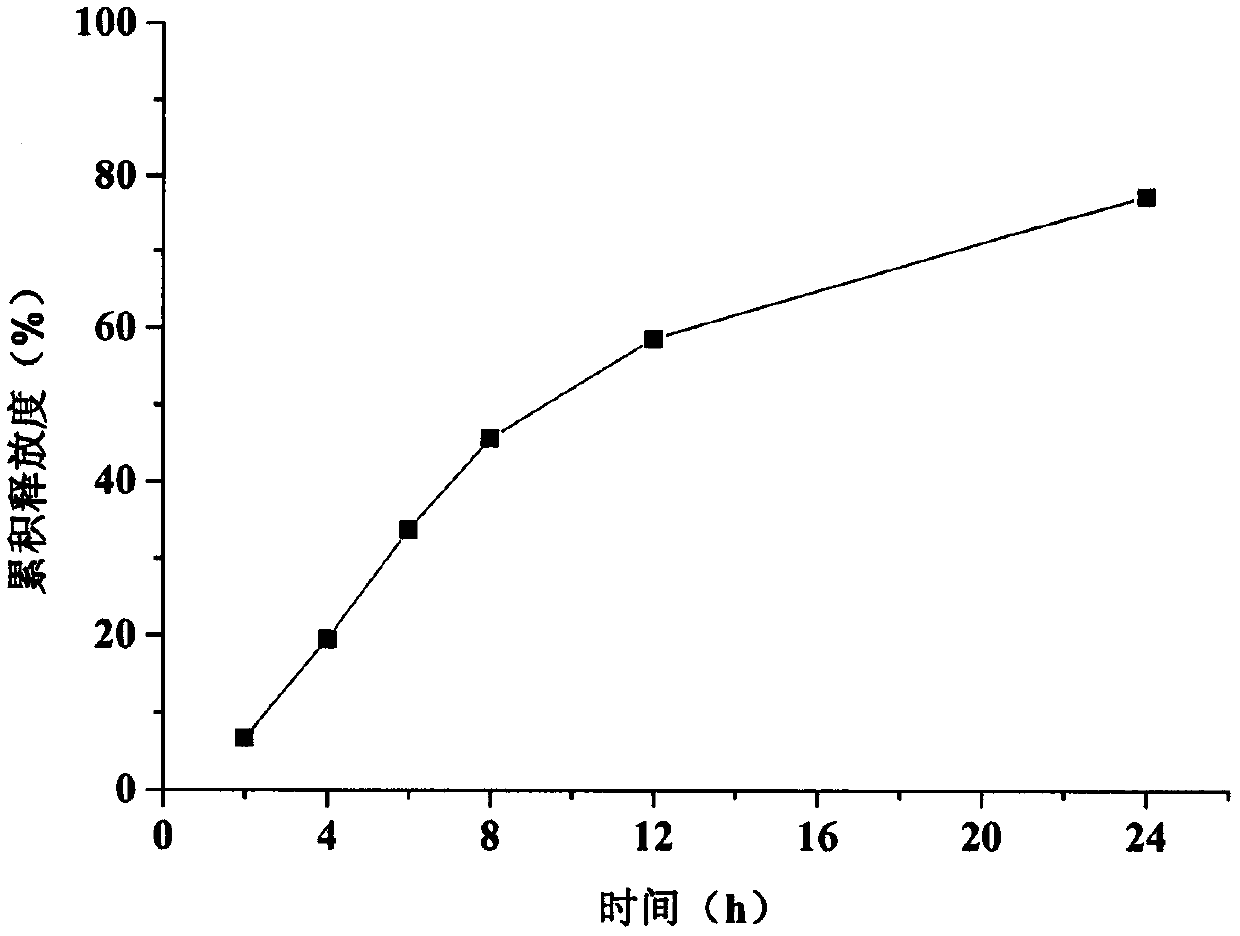
Examples
Embodiment 1
[0030] (1) Tablet core prescription (per tablet):
[0031]
[0032] (2) Composition of semi-permeable membrane coating solution (per 1000 tablets)
[0033]
[0034] Example 1 Preparation process of trimetazidine hydrochloride monolayer osmotic pump controlled-release tablet:
[0035] (1) Wet granulation: disperse trimetazidine hydrochloride and lactose through a 30-mesh sieve, then add hydroxyethyl cellulose and mix evenly, add 70% ethanol-water solution to moisten the soft material, pass through a 20-mesh sieve to granulate , dry at 40°C, control the water content below 3%, sieve through a 20-mesh sieve for granulation, add the prescribed amount of magnesium stearate and mix evenly;
[0036] (2) Tablet compression: a single-layer osmotic pump core is pressed with a circular shallow concave die with a diameter of 8 mm;
[0037] (3) Semi-permeable membrane coating: the above-mentioned tablet core was coated with a semi-permeable membrane coating solution, and the weight...
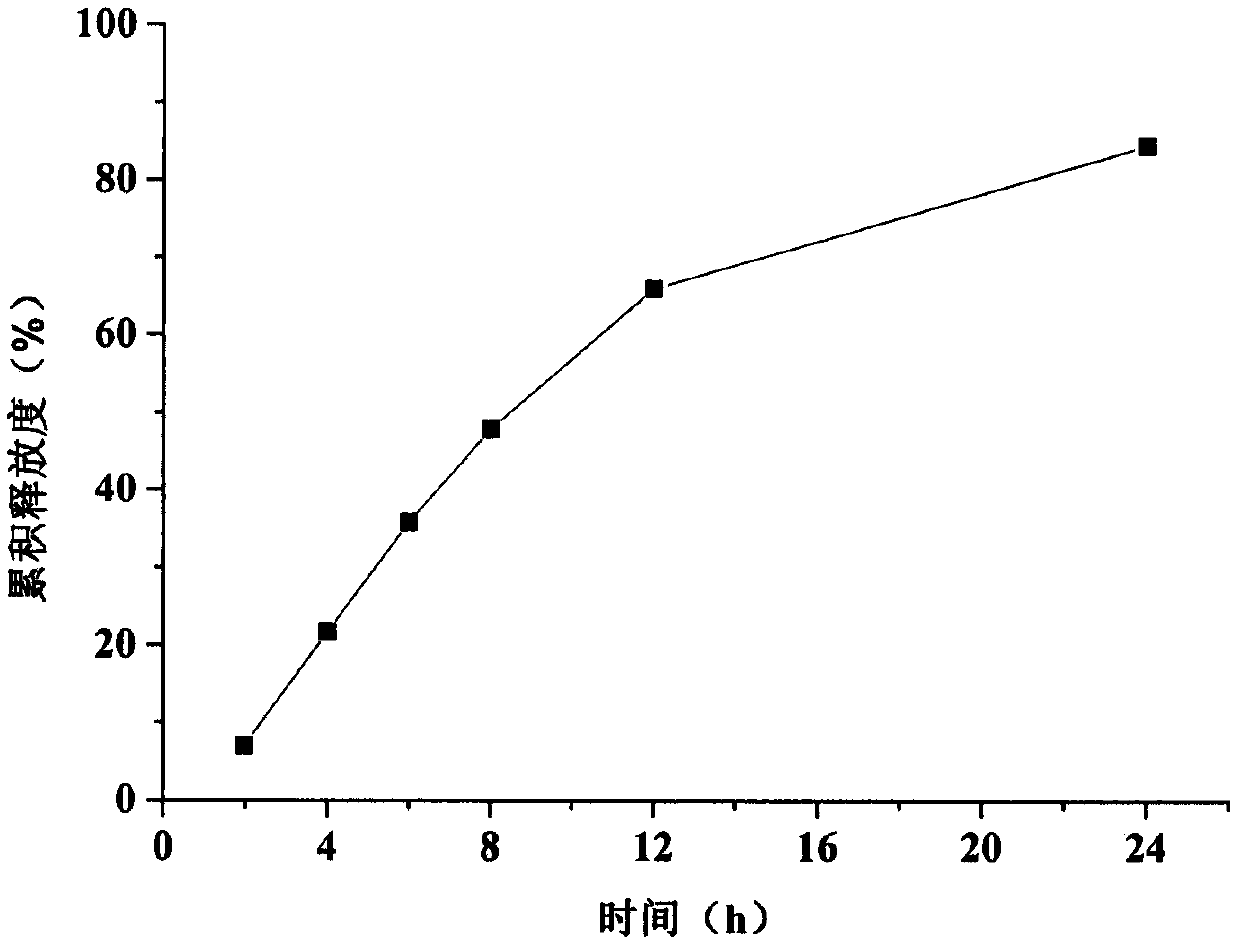
Embodiment 2
[0044] (1) Tablet core prescription (per tablet):
[0045]
[0046] (2) Composition of semi-permeable membrane coating solution (per 1000 tablets)
[0047]
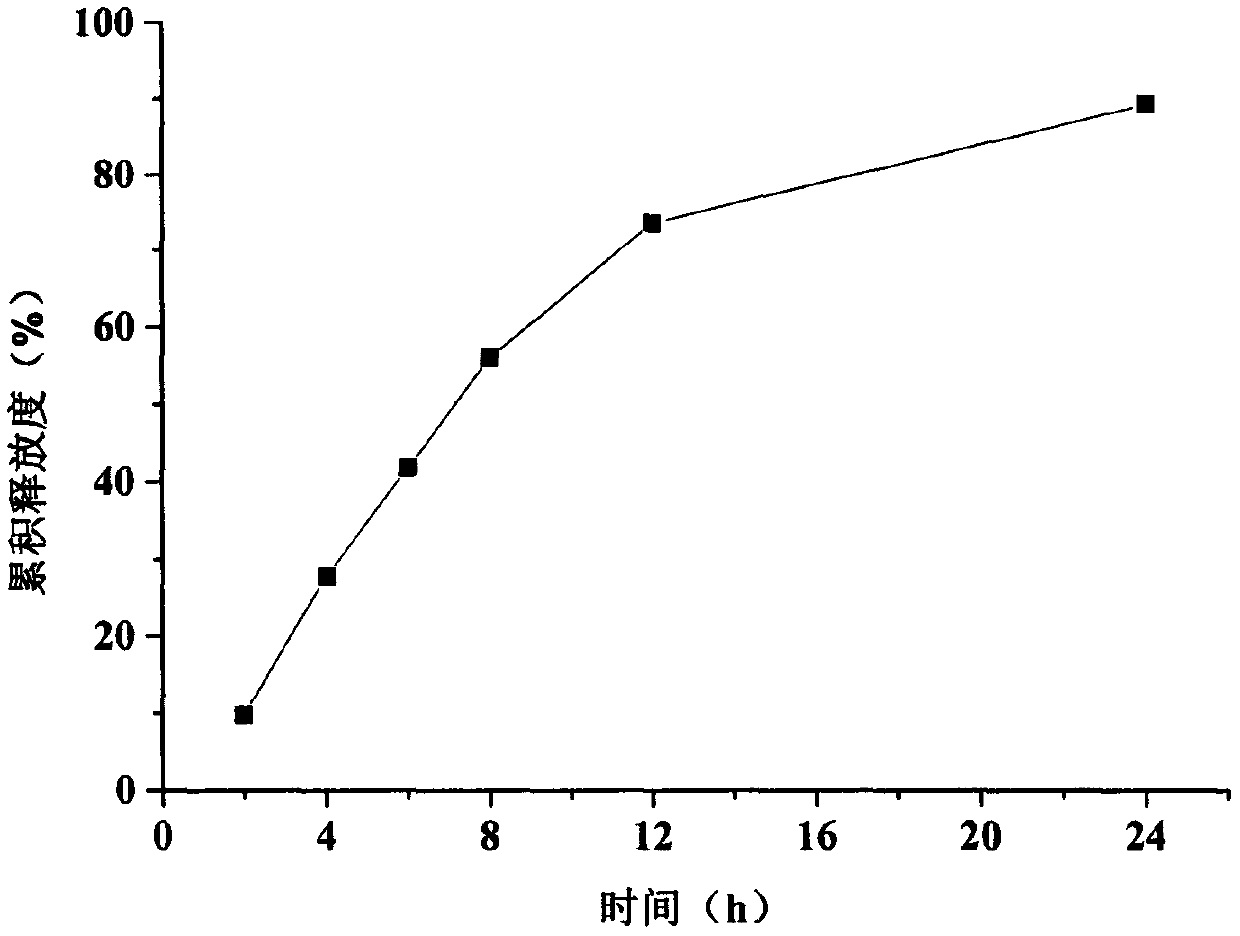
Embodiment 4
[0048] Embodiment 2-Example 4 Trimetazidine hydrochloride monolayer osmotic pump controlled-release tablet preparation process:
[0049] (1) Wet granulation: Sodium chloride is passed through a 50-mesh sieve, and lactose, mannitol, and trimetazidine hydrochloride are respectively passed through a 30-mesh sieve for dispersion. Mix trimetazidine hydrochloride and other auxiliary materials (except lubricant) in the tablet core evenly, add 70% ethanol-water solution to moisten the soft material, pass through a 20 mesh sieve to granulate, dry at 40°C, control the moisture below 3%, pass 20 mesh sieve for granulation, add the magnesium stearate of prescription quantity and mix evenly;
[0050] (2) Tablet compression: a single-layer osmotic pump core is pressed with a circular shallow concave die with a diameter of 8 mm;
[0051] (3) Semi-permeable membrane coating: the above-mentioned tablet core was coated with a semi-permeable membrane coating solution, and the weight gain of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com