A kind of trimetazidine hydrochloride osmotic pump controlled-release tablet and preparation method thereof
A controlled-release technology of trimetazidine hydrochloride and osmotic pumps, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, and medical preparations containing active ingredients, etc., which can solve the problem of unfavorable drug effects, short duration, and taking before bedtime The drug is in the body in the early morning, the risk of cardiovascular disease is high and the risk is unfavorable, so as to achieve the effect of reducing production cost and reducing the impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
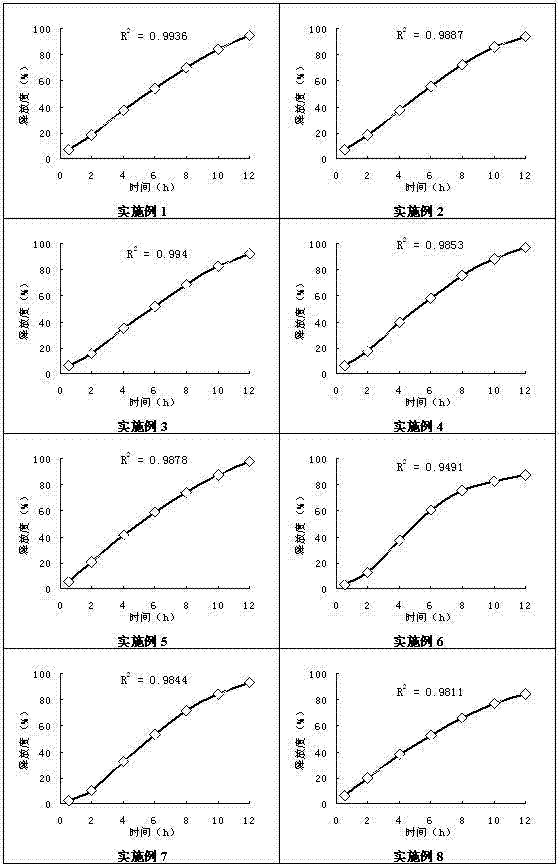
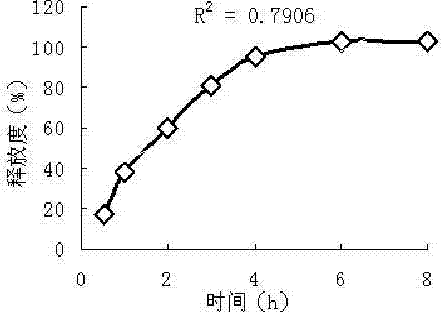
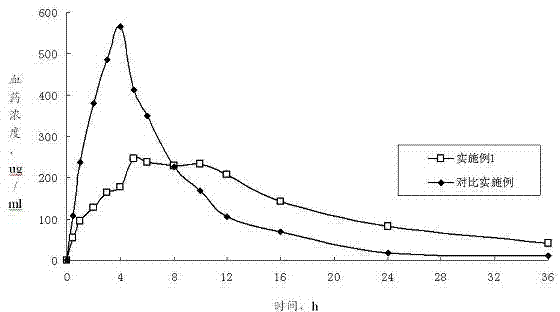
Examples
Embodiment 1
[0041] composition Dosage (mg) trimetazidine hydrochloride 35 microcrystalline cellulose 30 Polyethylene oxide (5 million) 5 Sodium chloride 45 lactose 45 dextrin 30 Povidone K30 10 Magnesium stearate 1
[0042] composition Dosage (mg) Cellulose acetate 20 Dibutyl phthalate 0.2 PEG-1500 4 acetone Appropriate amount
[0043] Preparation:
[0044] (1) After mixing the raw and auxiliary materials, moisten with absolute ethanol, granulate with 30 mesh, dry at 40°C, control the water content below 3%, and sieve with 24 mesh for granulation. Add the prescribed amount of magnesium stearate, and press the tablet with a shallow concave of φ8mm to obtain the osmotic pump tablet core.
[0045] (2) Coating liquid configuration: Take a certain amount of acetone, add cellulose acetate and PEG-1500 into the acetone solution, stir to dissolve; add plasticizer, stir and mix well. The soli...
Embodiment 2
[0050] composition Dosage (mg) trimetazidine hydrochloride 35 microcrystalline cellulose 80 Hydroxypropyl Methyl Cellulose (K100M) 20 Sodium chloride 30 dextrin 25 Povidone K30 10 Magnesium stearate 1
[0051] composition Dosage (mg) Cellulose acetate 20 Dibutyl phthalate 0.2 PEG-1500 4 acetone Appropriate amount
[0052] Preparation method: same as the preparation method in Example 1, and the coating weight gain is the same as that of Example 1.
Embodiment 3
[0054] composition Dosage (mg) trimetazidine hydrochloride 35 microcrystalline cellulose 40 Polyethylene oxide (5 million) 5 Hydroxypropyl Methyl Cellulose (K100M) 15 Sodium chloride 25 Mannitol 20 dextrin 80 Povidone K30 10 Magnesium stearate 1
[0055] composition Dosage (mg) Cellulose acetate 20 PEG-6000 5 acetone Appropriate amount
[0056] Preparation method: the same preparation method as in Example 1, using φ8.5mm shallow concave tablet compression, and the weight gain of the coating is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com