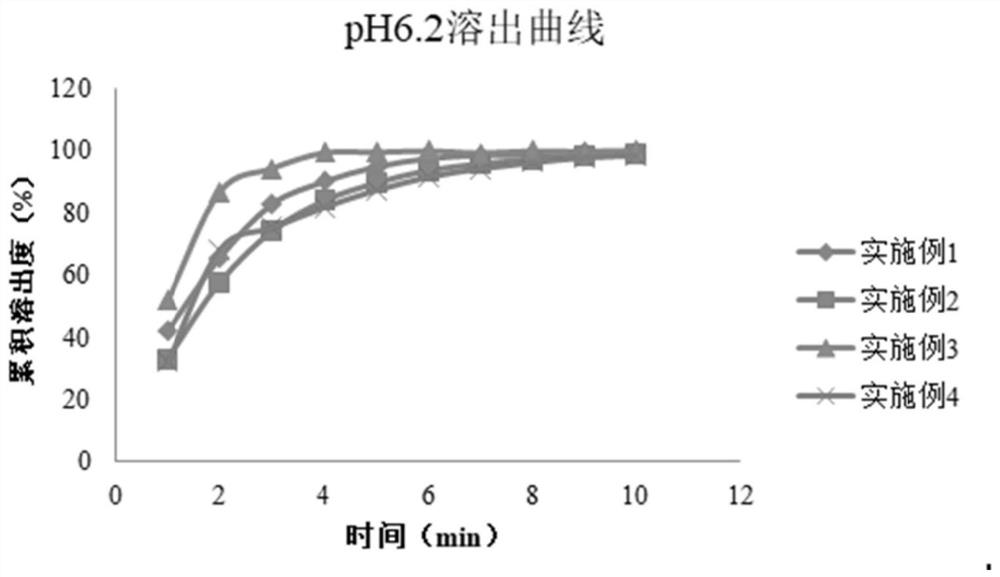
Trimetazidine hydrochloride sublingual tablet pharmaceutical preparation
A technology of trimetazidine hydrochloride and trimetzidine hydrochloride, applied in the field of sublingual tablets, can solve the problem of few types of drugs, and achieve the effects of fast disintegration and dissolution rate, good sample reproducibility, and simple process preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation Example
[0025]
[0026] Preparation Process:
[0027] (1) 2 parts of trimetazidine hydrochloride was mixed with 1.5 parts of sodium saccharin and 1.5 parts of tartaric acid, and then mixed with 68 parts of mannitol.
[0028] (2) Add 1 part of sodium carboxymethyl cellulose, 0.2 part of cherry essence and 0.8 part of sodium stearyl fumarate to the above mixed powder, and mix uniformly.
[0029] (3) Pressing the above-mentioned total powder with a 5mm round punch, the tablet weight is 75mg and the hardness is 2-3kg.
Embodiment 2
[0030] Example 2: Preparation Example
[0031]
[0032]
[0033] Preparation Process:
[0034] (1) 2 parts of trimetazidine hydrochloride was mixed with 1 part of sodium saccharin and 1 part of tartaric acid, and then mixed with 69.9 parts of mannitol.
[0035] (2) Add 3 parts of sodium carboxymethyl cellulose, 0.2 part of cherry essence and 1 part of sodium stearyl fumarate to the above mixed powder, and mix uniformly.
[0036] (3) Pressing the above-mentioned total powder with a 5mm round punch, the tablet weight is 75mg and the hardness is 2-3kg.
Embodiment 3
[0037] Example 3: Preparation Example
[0038]
[0039] Preparation Process:
[0040] (1) 2 parts of trimetazidine hydrochloride was mixed with 0.8 parts of sodium saccharin and 1.2 parts of tartaric acid, and then mixed with 65 parts of mannitol.
[0041] (2) Add 1 part of sodium carboxymethyl cellulose, 0.2 part of cherry essence and 0.8 part of sodium stearyl fumarate to the above mixed powder, and mix uniformly.
[0042] (3) Pressing the above-mentioned total powder with a 5mm round punch, the tablet weight is 75mg and the hardness is 2-3kg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com