Trimetazidine hydrochloride sustained-release tablet
A technology of trimetazidine hydrochloride and sustained-release tablets, which is applied in the direction of drug combination, cardiovascular system diseases, organic active ingredients, etc., can solve the problems of cumbersome preparation process steps, incomplete release, and unfavorable industrial production, and achieve the goal of preparing The effect of simple process, low production cost, and stable dissolution and release properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
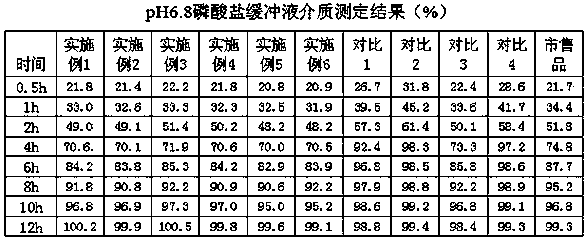
Examples
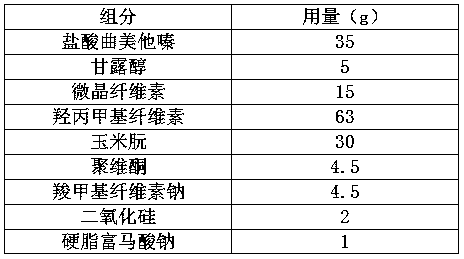
Embodiment 1
[0028]
[0029] Preparation:
[0030] A. trimetazidine hydrochloride, calcium hydrogen phosphate and povidone are mixed homogeneously to obtain a mixture;
[0031] b. Wet granulate the above mixture, and granulate to obtain drug granules;
[0032] c. Mix the above drug granules with hypromellose and zein evenly, and press into tablets.
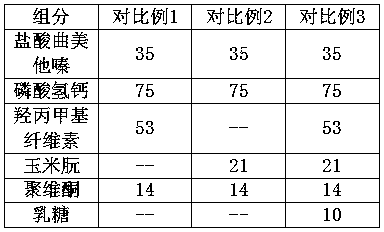
Embodiment 2
[0034]
[0035] Preparation:
[0036] a. mixing trimetazidine hydrochloride, microcrystalline cellulose, dextrin and sodium carboxymethyl cellulose to obtain a mixture;
[0037] b. Wet granulate the above mixture, and granulate to obtain drug granules;
[0038] c. Mix the above drug granules with hypromellose and zein evenly, and press into tablets.
Embodiment 3
[0040]
[0041] Preparation:
[0042] a. mixing trimetazidine hydrochloride, pregelatinized starch and povidone evenly to obtain a mixture;
[0043] b. Wet granulate the above mixture, and granulate to obtain drug granules;
[0044] c. Mix the above drug granules with hypromellose, corn gluten, magnesium stearate and silicon dioxide evenly, and press into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com